KNEC KCSE Chemistry Paper 3 – 2014 Cross Country Mock
2014 Cross Country Mock
Chemistry Paper 3
QUESTION 1 (24 Marks)
Answer all questions.
You are provided with the following;
(i) 2.1g of solid sodium carbonate solid W.
(ii) Hydrochloric acid solution Y
(iii) 0.2M sodium hydroxide, solution V
This question has two parts:
PART 1
- Measure 60cm3 of solution Y hydrochloric acid and transfer into a plastic beaker and measure its temperature T1,……………………………….……oC
- Take all the 2.1g sodium carbonate and transfer into the solution in the beaker. Stir with the thermometer and record final temperature reached, T2……………………………………0C ( ½ mk)
Keep the mixture for part II and label it X.
Calculations:
(a) Determine the rise in temperature (1mk)
∆t
(b) Determine the amount of heat evolved by the solution (density =1g/cm3, specific heat capacity of solution = 4.2kJKg-1K-1) (2mks)
(c) If the acid was in excess, determine the number of moles of sodium carbonate (Na = 23, O=16, H=1)(2mks)
(d) Calculate the number of moles of hydrochloric acid which reacts (2mks)
(e) Determine the molar heat of reaction of sodium carbonate (2mks)
PART II
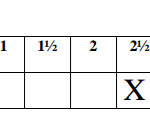
To the mixture in part I(X) add 20cm3 of distilled water and mix well. Transfer the solution in the burette.Pipette 25cm3 of NaOH, solution V, into the conical flask and titrate with solution X using phenolphthaleinindicator. Repeat the titration two more times and complete the table below:
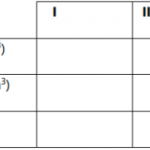
| Details | I | II | III |
| Final burette reading (cm3) |
|
|
|
| Initial burette reading (cm3) |
|
|
|
| Volume of X used (cm3) |
|
|
|
(i) Determine the average volume of X used (1mk)
(ii) Calculate the number of moles of NaOH in 25cm3 of solution V (2mks)
(iii) Determine the number of moles of hydrochloric acid that reacted with moles of 25cm3 of sodium
hydroxide (2mks)
(iv) Determine the number of moles of hydrochloric acid in 80cm3 of X (2mks)
(v) What is the total number of moles of hydrochloric acid in the original 60cm3 of HCL (1mk)
(vi) Hence determine the concentration of hydrochloric acid, solution Y in moles per litre (2mks)
26 marks
QUESTION 2 (14 Marks)
Answer all questions.
You are provided with solid N. carry out the tests below, write your observations and inferences in
the spaces provided.
| Test | Observation | Inferences |
| (a) Take a spatula endful of N in a test-tube and add distilled water until half-filled. Shake well and divide the solution into 5 portions |
(1mk) |
(1mk) |
| (b) To the first portion add 2M NaOH solution drop wise until in excess |
(2mk) |
(1mk) |
| (c)To the 2nd portion add 2M NH3(aq) drop wise until in excess |
(2mk) |
(1mk) |
| (d) To the 3rd portion add 3drops of 2M HCl solution |
(1mk) |
(1mk) |
| (e) To the 4th portion add about 1cm3 of 2M Pb(NO3)2 solution |
(1mk) |
(2mk) |
| (f) To the 5th portion add about 1cm3 of 2M Ba(NO3)2 solution followed by dilute nitric acid. |
(2mk) |
(1mk) |
14 marks