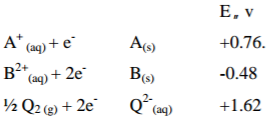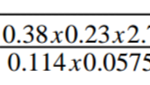KCSE Chemistry Paper 1 – 2014 EKSIKA Joint Evaluation Test
2014 EKSIKA Joint Evaluation Test
Chemistry Paper 1
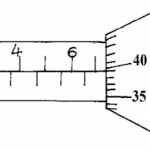
Two papers A and B were placed at different levels of a non-luminous flame. Paper A was placed on the lowest part of the flame while B was placed at the tip.
(a) Indicate below the observations made on each paper. (2mks)
(b) Explain the observations made on paper A. (1mk)
3 marks
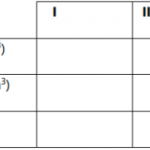
The table below shows the number of drops of soap solution needed to lather with 10cm33of water.
| Sample | Cold water | Heated water |
| A B C |
5
6 2 |
5
2 2 |
(a) Identify the anions likely to be in:
A (1mk)
B (1mk)
(b) State TWO methods used in removing permanent hardness of water.(1mk)
3 marks
20cm3 of sodium hydroxide solution containing 8.0g/dm3 were required for complete neutralization of 0.18g of a dibasic and H2X.Calculate the relative molecular mass of the acid.
3 marks
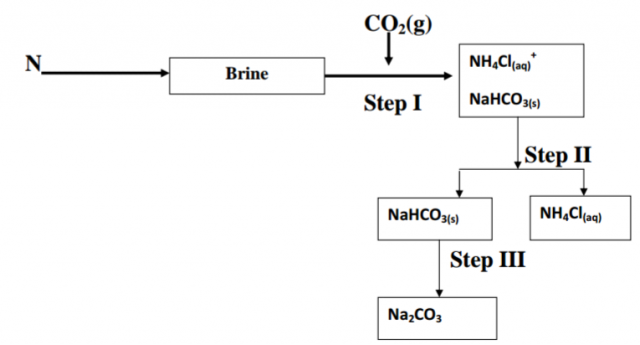
The flow chart below shows some of the stages in the manufacture of the sodium
carbonate by the Solvay process. Use it to answer the questions that follow;
(a) Name the substance N (1mk)
(b) Name the process taking place in.
(i) Step II (1mk)
(ii) Step III (1mk)
(c) Write an equation for the reaction producing Sodium Carbonate. (1mk)
4 marks
The solubility of copper (II) Sulphate at 75oC is 55g/100g of water and 19g/100g of water at 15oC. What mass of crystals would be deposited if a saturated solution in 150g of water is cooled from 75oC to 15oC.
3 marks
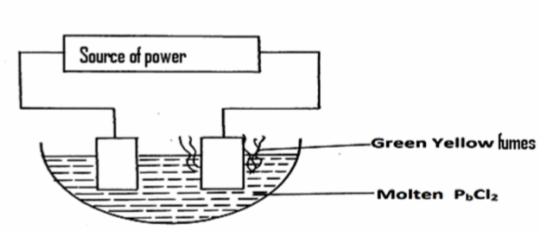
Use the set up below to answer the questions that follow.
(a) On the diagram label the cathode. (1mk)
(b) Write the equation of the reaction on the cathode. (1mk)
2 marks
(a)What is fuel? (1mk)
(b) Given that the enthalpy of combustion of methane is 890KJmol-1 and that of ethanol is 1368KJmol-1. Which of the two is a better fuel? Explain. (2mks)
3 marks
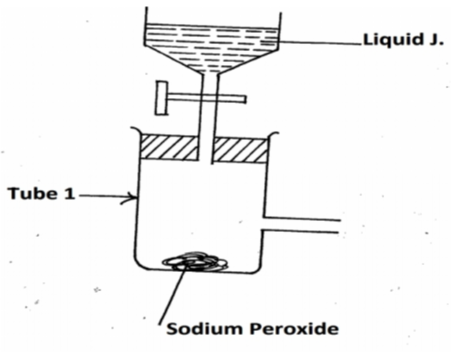
The diagram below represents parts of a set up for preparing and collecting a dry sample of oxygen gad.
(a) Complete the diagram. (1mk)
(b) Write the equation for the reaction in tube 1. (1mk)
(c) State ONE commercial use of the oxygen gas. (1mk)
3 marks
The table below shows some elements and their atomic numbers. The letters do not represent the actual symbols of the elements.
| Element | X | Y | Z | R | S | Q | T |
| Atomic Number | 11 | 10 | 20 | 13 | 14 | 4 | 8 |
(a) From the given letters of elements select two elements with the same chemical properties. (1mk)
(b) Write the formulae of a compound formed when element S reacts with
element T. (1mk)
(c) Identify the most stable element. (1mk)
2 marks
A dynamic equilibrium between dichromate and chromate ions is established as shown in the equation below.
(a) What is meant by a dynamic equilibrium. (1mk)
(b) State and explain the observation that would be made if a few pellets of sodium hydroxide are added to the equilibrium mixture. (2mks)
3 marks
The diagram below shows an experimental set up for preparing Carbon (II) Oxide. Study it and answer the questions that follow.
(a) State the role of sodium hydroxide solution in the set up. (1mk)
(b) State the reason why Carbon (II) Oxide is collected in the manner illustrated (1mk)
(c) Describe a simple test that can be used to distinguish between carbon (II)oxide and carbon (IV) oxide. (1mk)
3 marks
(a) State the Graham’s law of diffusion. (1mk)
(b) A sample of unknown compound Z is shown by analysis to contain sulphur and oxygen. The gas requires 28.3seconds to diffuse through aperture into a vacuum. An identical number of oxygen molecules pass through the same aperture in 20seconds.Determine the molecular mass of Z. (O=16,S=32). (2mks)
3 marks
Explain why aluminum chloride is fairly soluble in organic solvents while anhydrous
magnesium chloride is in soluble.
2 marks
(a) Define half-life of radioisotopes. (1mk)
(b) X grammes of a radioisotopes takes 100days to decay to 20g.If half-life of the same element is 25days, Calculate the initial mass X of the radioisotope. (2mks)
3 marks
Explain why the pH of 0.1M hydrochloric acid is 1.0 while that of 1.0M ethanoic acid is 5.0.
2 marks
Write down the property of concentrated Sulphuric (VI) acid shown in the following
reactions.
(a)
property:
(b) 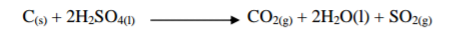
property:
2 marks
When excess chlorine gas is bubbled through dilute sodium hydroxide solution, the resulting solution acts as a bleeching agent.
(a) Write an equation for the reaction between chlorine gas and sodium hydroxide. (1mk)
(b) Explain how the resulting solution acts as a bleeching agent. (2mks)
3 marks
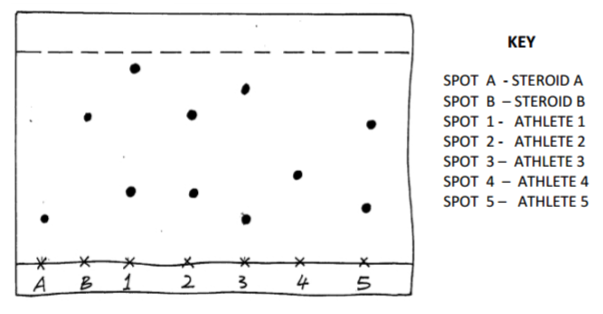
During Olympics, urine sample of five short distance runners were taken and tested for the presence of two illegal steroids by paper chromatography. Methanol was used as the solvent. A chromatogram from the test appeared as shown below. Study the chromatogram and answer question that follow.
(a) Which of the two steroids is most likely to be more soluble in methanol? Give a reason. (1mk)
(b) Identify the athletes that tested positive for the illegal steroids. (2mks)
3 marks
A carbonate was suspected to be an ore of iron. Describe how the presence of iron can
be confirmed in the ore.
3 marks
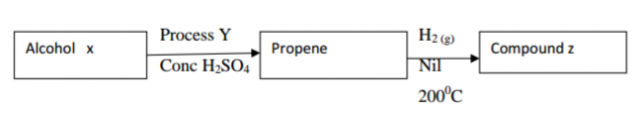
Use the reaction scheme below to answer the questions that follow.
(a) Draw the structure of alcohol X. (1mk)
(b) Name the process Y. (1mk)
(c) Write the molecular formula of the 5th member in which propene belongs. (1mk)
3 marks
Describe how a solid sample of Lead (II) Sulphate would be prepared using the following reagents. Dilute Sulphuric (VI) acid, Nitric (V) acid, solid lead (II)Carbonate.
3 marks
Give the following electrode potential.
(a) Determine the maximum E.M.F. that can be obtained by combining two
of the given half cells. (1mk)
(b) Write the cell representation for the cell in (a) above. (1mk)
(c) What would be the electrode potential of A if B was made standard electrode.(1mk)
3 marks
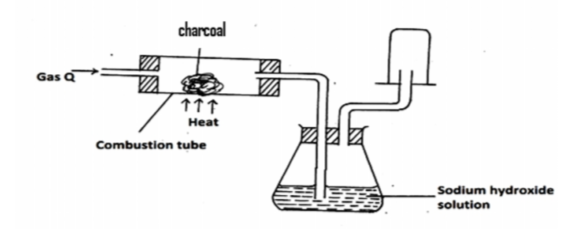
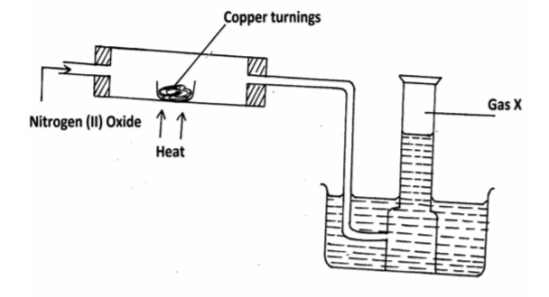
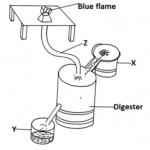
Study the set up below and answer the questions that follow.
(a) Identify gas X. (1mk)
(b) State the observation made in the combustion tube. (1mk)
(c) Write equation for the reaction in combustion tube(1 mk)
3 marks
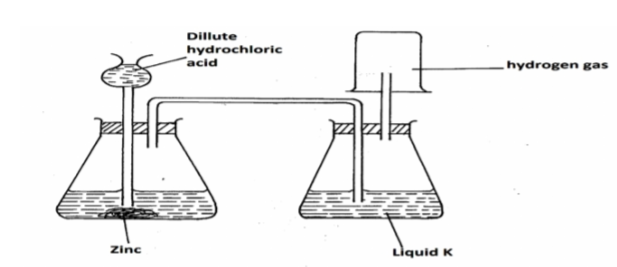
The diagram below represents an arrangement for preparing and collecting dry hydrogen. Study it and answer the questions that follow.
(a) Write the equation for the reaction that produces hydrogen gas. (1mk)
(b) Name the suitable substance that liquid K is likely to be. (1mk)
(c) Explain why it is not advisable to use nitric (v) acid as an alternative to
hydrochloric acid in the preparation experiment. (1mk)
3 marks
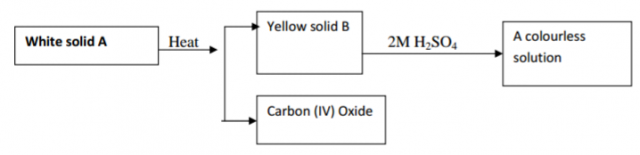
The scheme below represents some reactions starting with a white solid A.
(a) Identify the solids;
A (1mk)
B (1mk)
(b) Write an equation for the reaction between B and 2M Sulphuric acid.(1mk)
3 marks
Using dots (.) and ( x ) show bonding in:
(a) The compound formed when nitrogen reacts with fluorine (Atomic number F= 9 , N = 7) (2mks)
(b) Sodium oxide (Atomic number Na = 11 , O = 8 ) (1mk)
3 marks
An aide of potassium has a relative formula mass of 110 , if 2.75g of the oxide contains 1.95g of potassium ,determine the formula of the oxide. K = 39.0 , O = 16.0 )
3 marks
Explain what happens when blue litmus paper is dipped in methylbenzene in which
hydrogen chloride is bubbled.
2 marks
Give reason for use of aluminum in sufurias but not steam boilers.
2 marks