KNEC KCSE Chemistry Paper 1 Question Paper / 2016 KCSE KAMDARA JET Examination
2016 KCSE KAMDARA JET Examination
Chemistry Paper 1
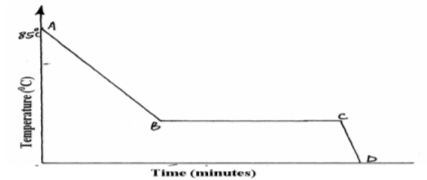
A Student in form four placed a thermometer in molten naphthalene at 85o C and recorded the
temperature and time until the naphthalene solidified. From the values obtained, the figure
below was drawn.
(a) What name is given to such a figure?…………………………………………………………….. (1mk)
(b) Which part of the figure represents the change of state of naphthalene?…………. (1mk)
(c) In terms of kinetic theory. Explain what happens to molecules along AB. (1mk)
……………………………………………………………………………………………
……………………………………………………………………………………………
……………………………………………………………………………………………
……………………………………………………………………………………………
3 marks
In a certain reaction, 18.7cm3 of a dibasic acid 2 XH required 25cm3 of 0.1M NaOH for
complete neutralization.
(a) How many moles of Sodium hydroxide are contained in 25cm3? (1mk)
……………………………………………………………………………………………
……………………………………………………………………………………………
……………………………………………………………………………………………
……………………………………………………………………………………………
……………………………………………………………………………………………
(b) Calculate the molarity of the dibasic acid. (2mks)
……………………………………………………………………………………………
……………………………………………………………………………………………
……………………………………………………………………………………………
……………………………………………………………………………………………
……………………………………………………………………………..
3 marks
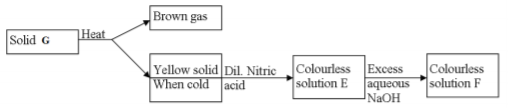
Study the flow chart below and answer the questions that follow.
(a) Identify solid G (1mk)
…………………………………………………………………………………………………
(b) Write a balanced chemical equation between the yellow solid and dilute nitric acid. (1mk)
………………………………………………………………………………………………………
………………………………………………………………………………………………………
……………………………………………………………………………………………………….
(c) Write the formula of the complex ion in solution F (1mk)
………………………………………………………………………………………………………
………………………………………………………………………………………………………
………………………………………………………………………………………………………
3 marks
Explain this observation:
When hydrogen chloride gas is dissolved in water, the solution conducts electricity while a
solution of hydrogen chloride gas in methyl benzene does not conduct electricity.
…………………………………………………………………………………………………
…………………………………………………………………………………………………
…………………………………………………………………………………………………
…………………………………………………………………………………………………
2 marks
Matter exists in three states which can be related as shown in the diagram below.
(a) Name processes: P: ——————————————————- (1mark)
R: —————————————————– (1mark)
(b) Explain whether process Q is exothermic or endothermic (1mark)
…………………………………………………………………………………………………
…………………………………………………………………………………………………
…………………………………………………………………………………………………
…………………………………………………………………………………………………
…………………………………………………………………………………………………
…………………………………………………………………………………………………
……………………………………
3 marks
(a) What is meant by allotropy? (1mark)
………………………………………………………………………………………………………
.……………………………………………………………………………………………………
(b) Name two allotropes of carbon. (1mark)
………………………………………………………………………………………………………
(c) Give one use of charcoal in the sugar refinery industry. (1mk)
………………………………………………………………………………………………………
3 marks
(a) State Graham’s Law of Diffusion (1mk)
………………………………………………………………………………………………………
………………………………………………………………………………………………………
(c) A given volume of ozone (O3) diffused from a certain apparatus in 96 seconds. Calculate the
time taken by an equal volume of carbon(IV) oxide to diffuse under the same conditions.
(C=12,O=16) (2mks)
………………………………………………………………………………………………………
………………………………………………………………………………………………………
………………………………………………………………………………………………………
………………………………………………………………………………………………………
3 marks
(a) Name two ores from which copper is extracted. (1mk)
…………………………………………………………………………………………………
…………………………………………………………………………………………………
(b)During the extraction of copper metal the ore is subjected to froth floatation. Give a reason
why this process is necessary. (1mk)
……………………………………………………………………………………………………………………………………
………………………………………………………………………………………………………………….
(d) One of the alloys of copper is brass. State its two uses. (1mk)
………………………………………………………………………………………………
……………………………………………………………………………………………….
3 marks
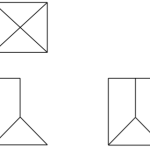
Draw a dot (● ) and cross (X) diagram to show bonding in sulphur (IV) oxide.
1 marks
A form one class carried out an experiment to determine the active part of air. The diagram
below shows the set-up of the experiment and also the observation made.
(a) Identify substance M ……………………………………… (1mk)
(b) State two reasons for the suitability of substance M for this experiment (1mk)
………………………………………………………………………………………………………
………………………………………………………………………………………………………
(c) Write the equation for the reaction of substance M and the active part of air (1mk)
……………………………………………………………………………………………………
3 marks
(a) Complete the following equation (1mk)
(b) Name the homologous series to which the following compounds belong?
(i) CH3CCH ……………………………………… (1mk)
(ii) CH3CH2OOCCH3 ………………………………………………… (1mk)
3 marks
The table below shows the pH values of solutions J to N
| Solution | J | K | L | M | N |
| pH | 5 | 13 | 2 | 10 | 7 |
(a) Which solution contains the largest concentration of hydroxides ions?………………..(1mk)
(b) Which solution is likely to be a solution of acetic acid?……………………………………….(1mk)
2 marks
The scheme below was used to prepare a cleansing agent. Study it and answer the questions
that follow.
(i) What name is given to the type of cleansing agent prepared by the method shown in the
scheme? (1mk)
…………………………………………………………………………………………………
(ii) Name one chemical substance added in step II (1mk)
…………………………………………………………………………………………………
(iii) What is the purpose of adding the chemical substance named in (ii) above. (1mk)
…………………………………………………………………………………………………
3 marks
a) Define half – life of radio isotopes. (1mk)
………………………………………………………………………………………………………
………………………………………………………………………………………………………
b) Z grammes of a radioactive isotope take 100 days to decay to 20gms. If the
half – life of the element is 25 days. Calculate the initial mass of Z of the radio- isotope. (2mks
………………………………………………………………………………………………………
………………………………………………………………………………………………………
………………………………………………………………………………………………………
3 marks
Magnesium was burnt in air forming a white residue T. When put in a boiling tube with
water effervescence was noticed and colourless gas D with a characteristic pungent smell
was evolved. The gas turned a wet red litmus paper blue.
(a) Identify
(i) Residue T …………………………………………………………….(1mk)
(ii) Gas D…………………………………………………………… .(1mk)
(b) Write an equation for liberation of gas D. ( 1mk)
…………………………………………………………………………………………
…………………………………………………………………………………………
3 marks
Explain why the bleaching action of chlorine is permanent while bleaching by sulphur (IV)
oxide is temporary.
…………………………………………………………………………………………………
…………………………………………………………………………………………………
………………………………………………………………………………………………
2 marks
Explain how you would separate a mixture of nitrogen and oxygen gases given that their
boiling points are -196oC and -183oC respectively.
……………………………………………………………………………………………………..
………………………………………………………………………………………………………
………………………………………………………………………………………………………
3 marks
Hydrazine gas, shown below, burns in oxygen to form nitrogen gas and steam.
(a) Write an equation for the reaction (1mk)
……………………………………………………………………………………………..
(b) Using the bond energies given below, calculate the enthalpy change for the reaction in (a)
above (2mks)
| Bond | Bond energy KJ per mole |
| N ≡ N | 944 |
| N=N | 163 |
| N−H | 388 |
| O =O | 496 |
| H−O | 463 |
…………………………………………………………………………………………………
…………………………………………………………………………………………………
…………………………………………………………………………………………………
…………………………………………………………………………………………………
…………………………………………………………………………………………………
3 marks
Using reagents provided only, explain how you could prepare solid Zinc carbonate.
- Zinc powder
- Nitric (V) acid (dilute)
- Water
- Solid sodium carbonate
………………………………………………………………………………………………………
………………………………………………………………………………………………………
………………………………………………………………………………………………………
………………………………………………………………………………………………………
2 marks
The apparatus below was set up to show the catalytic oxidation of ammonia.
(a) Identify the brown fumes observed at the mouth of the conical flask. (1mk)
…………………………………………………………………………………………
(b) Write down the equations of the reactions representing
(i) Catalytic oxidation of ammonia (1mk)
……………………………………………………………………………………
(ii) The formation of the brown fumes. (1mk)
……………………………………………………………………………………
3 marks
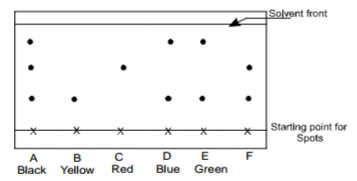
Consider the chromatogram below.
A piece of chromatogram paper was spotted with colour inks obtained from pens labeled A to
F. The diagram above shows the spots after the chromatograph was developed.
(a) Which two pens contained the same pigment?……………………………………………….. (1mk)
(b) According to the chromatogram which pigments are present in the inks of the pen number F
……………………………………………………………………………………… (1mk)
(c) Describe how one could get a sample of yellow pigment (1mk)
………………………………………………………………………………………………
………………………………………………………………………………………………
………………………………………………………………………………………………
………………………………………………………………………………………………
3 marks
Consider the following reaction at equilibrium.
PCl5(g) ⇌ PCl3(g) +Cl2 (g)
Complete the table below to show the effect of different factors on the position of equilibrium.
3 marks
A student investigated the effect of an electric current by passing it through some substances.
The student used inert electrodes and connected a bulb to the circuit. The table below shows
the substances used and their states.
| Experiment | Substance | State |
| 1 | Potassium carbonate | Solid |
| 2 | Copper (II) sulphate | Solution |
| 3 | Sugar | Solution |
| 4 | Lead (II) iodide | Molten |
(a) In which experiments did the bulb not light? ………………………………….. (1mk)
(b) Explain your answer in (a) above. (2mks)
………………………………………………………………………………………………
………………………………………………………………………………………………
……………………………………………………………………………………………..
3 marks
Give a reason why the formula mass of NO2 is sometimes 92 instead of 46.
…………………………………………………………………………………………………
…………………………………………………………………………………………………
…………………………………………………………………………………………………
…………………………………………………………………………………………………
1 marks
A compound contains only carbon, hydrogen and oxygen .Combustion of 1.068g of the
compound produces 1.601g of carbon (IV) oxide and 0.437g of water. The molar mass of the
compound is 176.1g⁄mol. What is the empirical and molecular formulae of the compound?
………………………………………………………………………………………………
………………………………………………………………………………………………
………………………………………………………………………………………………
………………………………………………………………………………………………
………………………………………………………………………………………………
………………………………………………………………………………………………
………………………………………………………………………………………………
………………………………………………………………………………………………
………………………………………………………………
2 marks
(a) A sample of water in a beaker was found to boil at 102℃ at 1 atmospheric pressure.
Assume that the thermometer was not faulty explain this observation (1mk)
…………………………………………………………………………………………………
…………………………………………………………………………………………………
(b)Study the information in the table below and answer the questions that follow.
| Salt | Solubility (g/100g water) At 40℃ | Solubility (g/100g water) At 60℃ |
| CuSO4 | 28 | 38 |
| Pb(No3)2 | 79 | 98 |
A mixture containing 35g of CuSO4 and 78g of Pb(NO3)2 in 100g of water at 60℃
was cooled to 40℃
(i) Which salt crystallized out? Give a reason. ……………………………… (1mk)
(ii) Calculate the mass of the salt that crystallized out. (1mk)
…………………………………………………………………………………
…………………………………………………………………………………
…………………………………………………………………………
3 marks
A student was asked to determine the percentage of zinc metal in a mixture of zinc metal and
zinc oxide. He reacted the mixture with excess hydrochloric acid and accurately collected the
gas evolved, which was then used to calculate the amount of zinc in the mixture.
(a) Name the gas that was evolved………………………………………………… (1 mark)
(b) Apart from the reaction liberating the gas write a balanced equation for the other reaction
that took place . (1 mark)
…………………………………………………………………………………………
…………………………………………………………………………………………
………………………………………………………………………………………..
(c) Why would dilute nitric acid not suitable for this reaction? (1 mark)
……………………………………………………………………………………………
……………………………………………………………………………………………
……………………………………………………………………………………………
……………………………………………………………………………………………
…………………………………
3 marks
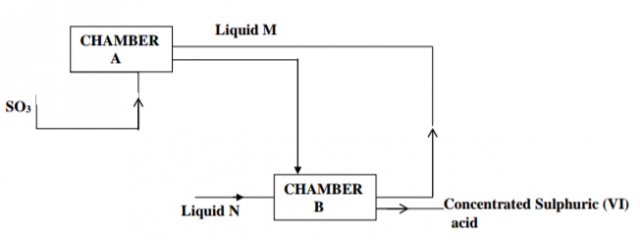
Below is part of the flow diagram of the contact process.
(a) Identify (i) Liquid M………………………………………………… (1mk)
(ii) Liquid N………………………………………………… (1mk)
(b) Write the equation for the reaction taking place in chamber B. (1mk)
…………………………………………………………………………………………
…………………………………………………………………………………………
……………………………………………………………………………..
3 marks
Chlorine gas dissolved in distilled water to form chlorine water
(a) Name the compounds present in the chlorine water. ………………………………… (1mk)
(b) What would be observed if blue litmus paper is dipped in chlorine water? Explain. ( 2mks)
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
3 marks
A fixed mass of gas occupies 105cm3 at -14℃ and 650mmHg pressure. At what temperature
will it have a volume of 15cm3 if the pressure is adjusted to 690 mmHg pressure
…………………………………………………………………………………………………
…………………………………………………………………………………………………
…………………………………………………………………………………………………
…………………………………………………………………………………………………
…………………………………………………………………………………………………
2 marks