KNEC KCSE Chemistry Paper 2 Question Paper / 2016 KCSE MOKASA Joint Examination
2016 KCSE MOKASA Joint Examination
Chemistry Paper 2
The grid below shows part of the periodic table. Use it to answer the questions that follow.
(The letters do not represent the actual symbols.)
a) Which of the above elements has the largest atomic radius? Explain? (1 mark)
………………………………………………………………………………………………….
………………………………………………………………………………………………….
………………………………………………………………………………………………….
b) Identify the most reactive non-metal. Explain (1 mark)
………………………………………………………………………………………………….
………………………………………………………………………………………………….
………………………………………………………………………………………………….
c) Write the electron configuration of ions of;
(i) Element S (½ mark)
………………………………………………………………………………………………….
………………………………………………………………………………………………….
(ii) Element Q (½ mark)
………………………………………………………………………………………………….
………………………………………………………………………………………………….
d) Compare the atomic radius of P and R (1 mark)
………………………………………………………………………………………………….
………………………………………………………………………………………………….
e) Write the formula of one stable cation with an electron arrangement of 2:8 (1 mark)
………………………………………………………………………………………………….
………………………………………………………………………………………………….
………………………………………………………………………………………………….
f) Given that the atomic mass of W is 40 write down the composition of its nucleus
(1 mark)
………………………………………………………………………………………………….
………………………………………………………………………………………………….
………………………………………………………………………………………………….
g) Write the formula of the compound formed when P and S react (1 mark)
………………………………………………………………………………………………….
………………………………………………………………………………………………….
………………………………………………………………………………………………….
h) Give the family to which element R belong (1 mark)
………………………………………………………………………………………………….
………………………………………………………………………………………………….
i) Element X forms an ion with the fomula X3- with electron configuration of 2.8. On the
grid above, show the position of element X (1 mark)
j) Compare the electrical conductivity of the compound formed between P and U and
element Q (2 marks)
………………………………………………………………………………………………….
………………………………………………………………………………………………….
………………………………………………………………………………………………….
………………………………………………………………………………………………….
11 marks
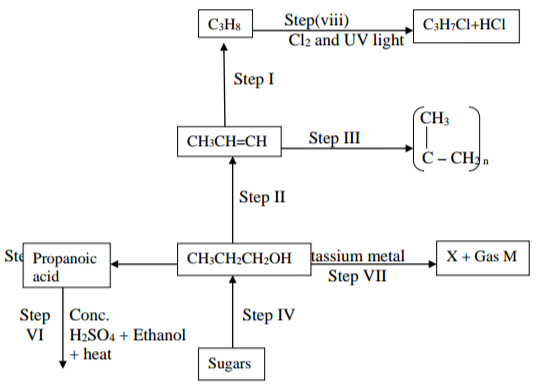
Study the flow chart below and answer the questions that follow.
a)i) Name the type of reaction in the following steps
Step I ……………………………………………………………………………………………… (1 mark)
Step IV ………………………………………………………………………………………….. (1 mark)
ii) Name the important reagents and conditions in;
Step I: (½ mark)
Reagent ……………………………………………………………………………………….
Condition …………………………………………………………………………………….
Step V: (½ mark)
Reagent …………………………………………………………………………… (½ mark)
Condition …………………………………………………………………………. (½ mark)
b) Name and draw the structure of compound L (2 marks)
………………………………………………………………………………………………….
………………………………………………………………………………………………….
c) Write an equation for the reaction in Step VII (1 mark)
………………………………………………………………………………………………….
………………………………………………………………………………………………….
d) State the homologous series to which the compound C3H8 belongs (1 mark)
………………………………………………………………………………………………….
………………………………………………………………………………………………….
………………………………………………………………………………………………….
e) State one industrial application of the process in Step I (1 mark)
………………………………………………………………………………………………….
………………………………………………………………………………………………….
f) Explain how one can distinguish between the compounds C3H8 and C3H6 using a
chemical test (2 marks)
………………………………………………………………………………………………….
………………………………………………………………………………………………….
………………………………………………………………………………………………….
………………………………………………………………………………………………….
11 marks
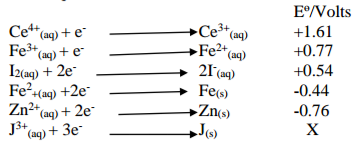
The following information show standard electrode potentials for some half reactions. Use it
to answer the questions that follow.
a) Identify the strongest reducing agent (1 mark)
………………………………………………………………………………………………….
………………………………………………………………………………………………….
b) Which substance in the table is suitable to oxidize iodide ions to iodine? (1 mark)
………………………………………………………………………………………………….
………………………………………………………………………………………………….
c) Study the cell representation below and answer the questions that follow.
Zn(s) /Zn2+(aq) // KNO3 Fe2+(aq) /Fe(s)
(i) Identify the anode and the cathode
Anode: ……………………………………………………………………………………………. (1 mark)
Cathode: …………………………………………………………………………………………. (1 mark)
(ii) If the two half cells in c(i) above are connected externally, write an equation taking
place in zinc half cell (1 mark)
………………………………………………………………………………………………….
………………………………………………………………………………………………….
………………………………………………………………………………………………….
iii) Calculate the e.m.f. of the cell (1 mark)
………………………………………………………………………………………………….
………………………………………………………………………………………………….
………………………………………………………………………………………………….
iv) State the role of KNO3 (1 mark)
………………………………………………………………………………………………….
………………………………………………………………………………………………….
………………………………………………………………………………………………….
v) Explain what happens when KCl(aq) is used instead of KNO3 in a case where
Pb(s)/Pb2+(aq)is one of the half cells (2 marks)
………………………………………………………………………………………………….
………………………………………………………………………………………………….
………………………………………………………………………………………………….
………………………………………………………………………………………………….
vi) Draw an electrochemical cell to represent the cell in c(ii) above (2 marks)
vii) If the e.m.f. of the cell J(s)/J3+(aq)//I2(s)/2I–(aq) is +1.32V, calculate the value of J3+(aq)/J(s)
(1 mark)
8 marks
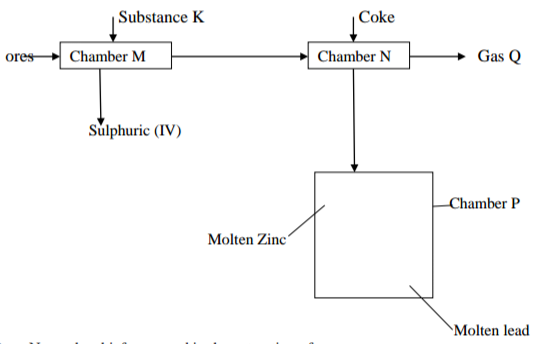
The flow diagram shows the extractions of lead and zinc metals. Study it and answer the
questions that follow.
a)i) Name the chief ores used in the extraction of;
Zinc (½ mark)
………………………………………………………………………………………………….
Lead (½ mark)
………………………………………………………………………………………………….
ii) Identify substances K and Q
K …………………………………………………………………………………… (½ mark)
Q …………………………………………………………………………………… (½ mark)
iii) State the function of coke in chamber N (1 mark)
………………………………………………………………………………………………….
………………………………………………………………………………………………….
iv) Write a chemical equation for the reaction between gas Q and calcium hydroxide
solution (1 mark)
………………………………………………………………………………………………….
………………………………………………………………………………………………….
v) What property makes it possible to separate the two metals (1 mark)
………………………………………………………………………………………………….
………………………………………………………………………………………………….
vi) Explain why zinc is preferred for coating iron to copper (1 mark)
………………………………………………………………………………………………….
………………………………………………………………………………………………….
vii) State two effects that this process would have on the environment (2 marks)
………………………………………………………………………………………………….
………………………………………………………………………………………………….
………………………………………………………………………………………………….
………………………………………………………………………………………………….
viii) Give one use of zinc (1 mark)
………………………………………………………………………………………………….
………………………………………………………………………………………………….
b) The process of obtaining pure zinc is by electrolytic method.
(i) Name the electrolyte used in the electrolytic method (1 mark)
………………………………………………………………………………………………….
………………………………………………………………………………………………….
………………………………………………………………………………………………….
………………………………………………………………………………………………….
(ii) Describe an experiment carried out to determine the presence of zinc metal in a
sample of soil using dilute sulphuric (vi) acid and aqueous ammonia (2 marks)
………………………………………………………………………………………………….
………………………………………………………………………………………………….
………………………………………………………………………………………………….
11 marks
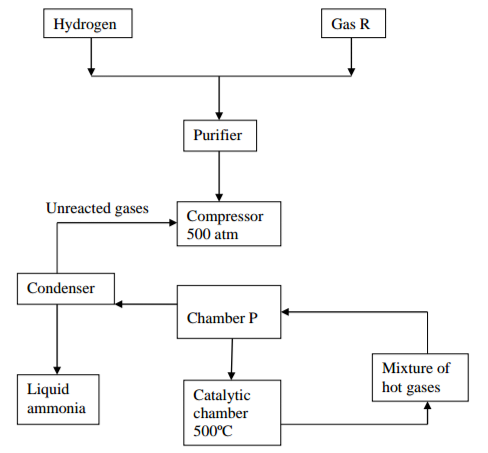
The flow chart below show the large scale manufacture of ammonia by haber process. Study
it and answer the questions that follow.
a) Identify gas R (1 mark)
………………………………………………………………………………………………….
b) i) Name two sources of hydrogen gas used in the process (2 marks)
………………………………………………………………………………………………….
………………………………………………………………………………………………….
………………………………………………………………………………………………….
ii) Explain the reason why the mixture of hydrogen gas and gas R are passed through the purifier
(2 marks)
………………………………………………………………………………………………….
………………………………………………………………………………………………….
iii) Name a suitable catalyst used in the catalystic chamber (1 mark)
………………………………………………………………………………………………….
………………………………………………………………………………………………….
c)i) Identify chamber P (½ mark)
………………………………………………………………………………………………….
ii) Explain why mixture of hot gases is passed through chamber P (1 mark)
………………………………………………………………………………………………….
………………………………………………………………………………………………….
………………………………………………………………………………………………….
iii) Write an equation for the main reaction in the catalytic chamber (1 mark)
………………………………………………………………………………………………….
………………………………………………………………………………………………….
d) Explain using equations the following observation
Hot platinum wire glows on coming into contact with fumes of Ammonia (2 marks)
………………………………………………………………………………………………….
………………………………………………………………………………………………….
………………………………………………………………………………………………….
………………………………………………………………………………………………….
e) State two industrial uses of ammonia (1 mark)
………………………………………………………………………………………………….
………………………………………………………………………………………………….
11 marks
a) Define the term molar heat of formation (1 mark)
………………………………………………………………………………………………….
………………………………………………………………………………………………….
b) Use the following standard enthalpies of combustion to answer the questions that follow.
ΔHөc (carbon) = -393kJmol-1
ΔHөc(H2g) = -286kJmol-1
ΔHc(C4H10) = -1290kJmol-1
(i) Write the equation for the formation of butane (1 mark)
………………………………………………………………………………………………….
………………………………………………………………………………………………….
(ii) Draw an energy level diagram that links heat of formation of butane with its heat of
combustion and the heats of combustion of carbon and hydrogen (3 marks)
(iii) Calculate the standard heat of formation of butane (2 marks)
………………………………………………………………………………………………….
………………………………………………………………………………………………….
………………………………………………………………………………………………….
………………………………………………………………………………………………….
c) Determine the heating value of butane (1 mark)
………………………………………………………………………………………………….
………………………………………………………………………………………………….
d) Use the bond energies below to calculate the enthalpy change for the formation of chloromethane
from methane gas and chlorine gas
| Bond H-Cl Cl-Cl C-H |
Bond energy in kJmol-1 431 242 413 |
(3 marks)
11 marks
I. The solution in grams of sodium nitrate in 100g of water is given for various temperature in degree
celcius
| Temperature | 0 | 10 | 20 | 30 | 40 | 50 | 60 | 80 | 90 | 100 |
| Solubility | 73 | 80 | 88 | 96 | 104 | 114 | 124 | 148 | 162 | 180 |
a) Draw a graph of solubility of sodium nitrate against temperature (3 marks)
b) From the graph determine the solubility of sodium nitrate at70ºC (1 mark)
c) 100 grams of a saturated solution of sodium nitrate at was cooled from 80ºC to 10ºC. What
mass will crystallize out (2 marks)
………………………………………………………………………………………………….
………………………………………………………………………………………………….
………………………………………………………………………………………………….
………………………………………………………………………………………………….
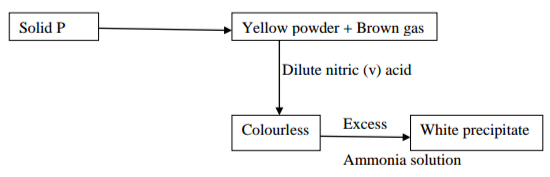
II Study the flow chart below and answer the questions that follow.
(i) Write the chemical formula of;
a) Solid P (1 mark)
………………………………………………………………………………………………….
………………………………………………………………………………………………….
b) The white precipitate (1 mark)
………………………………………………………………………………………………….
………………………………………………………………………………………………….
III Starting with copper(II) carbonate, describe how a solid sample of copper (II) sulphate
crystals would be prepared (3 marks)
11 marks