KCSE Chemistry Paper 2 – 2014 EKSIKA Joint Evaluation Test
2014 EKSIKA Joint Evaluation Test
Chemistry Paper 2
The grid below represents part of the periodic table. Study it and answer the questions that follow. The letters do not represent the actual symbols of the elements.
(a) What name is given to the family of the;
(i) Elements to which E, H and M belong? (1mk)
(ii) Elements to which F,J and K belong? (1mk)
(b) Write the chemical formula of the;
(i) Sulphate of T. (1mk)
ii) Nitrate of J. (1mk)
(c) Name the type of bond and structure formed between reactions of:
(i) D and N. (1mk)
Bond
Structure
(ii) T and H. (1mk)
Bond
Structure
(d)( i)Ionic radius of element E is bigger than its atomic radius. Explain (2mks)
(ii) The Oxide of G has a lower melting point than the Oxide of L.Explain. (1mks)
(iii) Explain in terms of bonding and structure the following observation:
There is an increase in melting and boiling points from W to T.(2mks)
(e) Using dot (∙) and cross (X) diagram show bonding in ZV+4.(2mks)
8 marks
(a) The diagram below represents the extraction of sulphur from its underground deposits by the Frasch process. Study the diagram and answer the questions that follow.
(i) Name the substances that pass through pipes L,M and N. (3mks)
(ii) What is the purpose of the:
(i) Superheated water. (1mk)
(ii) Hot compressed air. (1mk)
(b) The flow chart below shows how sulphuric acid is produced on a large scale by contact process.
(i) Identify:
(i) Gas A……………………………………………………… (1mk)
ii) Solid B……………………………………………………….(1mk)
iii) Gas C………………………………………………………..(1mk)
iv) Substance E…………………………………………………(1mk)
(ii) Name the catalyst used in the reaction chamber. (1mk)
(iii) Write a chemical equation for the reaction taking place in the dilution chamber. (1mk)
(c) State one industrial use of sulphuric acid. (1mk)
12 marks
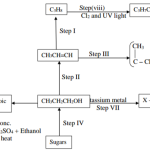
Study the flow chart below and answer the question that follows:
(a) i) Name the type of reaction in the following steps.
I. Step III ( ½ mk) ………………………………………………………………………………………………
II Step IV ( ½ mk) ………………………………………………………………………………………………
(ii) Name the important reagent and conditions in.
Step I Reagent………………………………………….. ( ½ mk)
Condition………………………………………… ( ½ mk)
Step II Reagent………………………………………….. ( ½ mk)
Condition………………………………………… ( ½ mk)
Step V Reagent…………………………………………… ( ½ mk)
Condition………………………………………… ( ½ mk)
(b) (i) Write a balance equation for the reaction taking place in VII(1mk)
(ii) Give the systematic name of substance L. (1mk)
(c) Describe chemical tests used to differentiate between C3H8 and C3H6.(2mks)
(d) (i) If the relative molecular mass of compound formed in step III is 42,000.Determine the value of n in the compound.(C=12.0 , H=1.0) (2mks)
(ii) State one disadvantage of continued use of items made from the
compound formed in stepIII. (1mk)
11 marks
Solubility of potassium and copper II Sulphate were determined at different
temperatures. The following data was obtained.
| Temperature (oC) | 0 | 20 | 40 | 60 | 80 | 100 |
| Solubility in 100g of water – KNO3 | 12 | 30 | 75 | 125 | 185 | 250 |
| Solubility in 100g of water – CuSO4 | 15 | 20 | 35 | 45 | 65 | 80 |
(i) On the graph paper provided; plot solubility curves for both salts, where solubility
(vertical axis) is plotted against temperature. (4mks)
(ii) Determine from the graph the solubility of each salt at 500C (1mk)
I. KNO3:
II. CuSO4 (1mk)
(iii) At what temperature was the solubility of both salts equal? (1mk)
(iv) Saturated solution of potassium nitrate at 700C was cooled to 200C.What mass
of the crystals will be deposited? (1mk)
(b) (i) What is permanent hardness of water? (1mk)
(ii) State two chemical substances that can be used to remove permanent hardness. (1mk)
(c) Explain why aluminum sulphate solution is acidic. (1mk)
11 marks
(a) Use the bond energies given in the table below to calculate the enthalpy
change for the reaction. (2mks)
| V | C – H | C –Br | Br – Br | H – Br | C-C |
| Bond energy KJ/mol | 413 | 280 | 193 | 365 | 343 |
C2H6 (g) + Br2 (g) →C2H5Br(g) + HBr(g)
(b) Hydrogen peroxide decomposes according to the equation below;
H2O(l) →H2O(l) + ½ O2(g) ; ∆H = -98KJ/mol.
If 6.8g of hydrogen peroxide contained 75cm3 of solution with water were completely decomposed, determine the rise in temperature due to the reaction.(Specific heat capacity of water =4.2Jg-1K-1, density of water =1g/cm3, O = 16 , H = 1). (2mks)
(c) On the space provided below sketch the cooling curve that would be obtained when a boiling tube containing water at 80oC is immersed in a freezing mixture maintained at -10oC. (3mks)
(d) Butane C4H10 cannot be prepared directly from its elements but its standard heat of formation (∆H”f) can be obtained directly. The following heats of combustion are given.
H”c carbon (s) = -393KJ/mol
H”c H2(g) = -286KJ/mol
H”c C4H10 = -2877KJ/mol
(i) Draw an energy circle diagram linking the heat of formation of butane with its heat of combustion and the heat of combustion of its constituents elements. (2mks)
(ii) Calculate the heat of formation of butane ∆H”f(C4H10). (2mks)
(e) Given that the lattice enthalpy of potassium chloride is +690KJ/mol and hydration enthalpies of K+ and Cl– are -322KJ and -364KJ respectively.
Calculate the enthalpy of solution of potassium chloride. (2mks)
13 marks
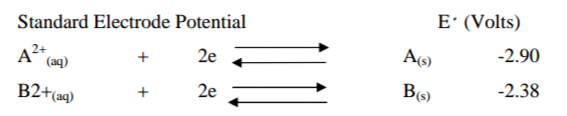
(a) Use the standard electrode potentials for elements A,B,C,D and E given below
to answer the questions that follow. The letters do not represent the actual
symbols of the elements.
(i) Which element is likely to be hydrogen?Give a reason for your
answer. (2mks)
(ii) What is Evalue of the strongest reducing agent? (1mk)
(iii) In the space provided, draw a labeled diagram of the electrochemical cell that would be obtained when half – cell of element B and D are!combined. (3mks)
(iv) Calculate the Evolume of the electrochemical cell constructed in (iii) above. (1mk)
(b) During the electrolysis of aqueous copper (II) Sulphate using copper electrodes, a current of 0.2A was passed through the cell for 5hrs.
(i) Write an ionic equation that took place at the anode. (1mk)
(ii) Determine the change in mass of the anode which occurred as a result
of the electrolysis process. (Cu = 63.5 , IF = 96500C). (3mks)
11 marks
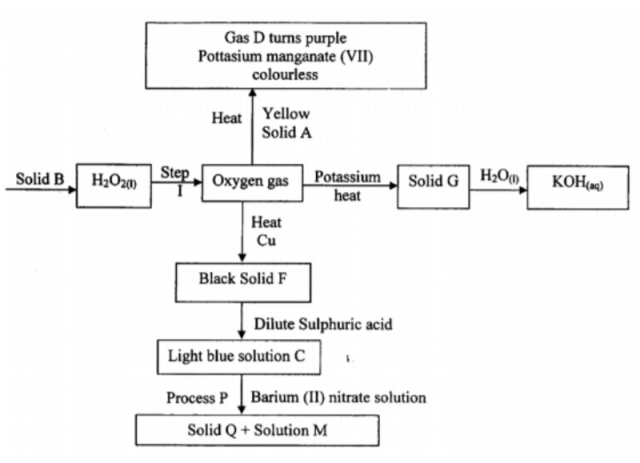
The flow chart below represents preparation and properties of oxygen gas.Study it and
answer the question that follow.
(i) Identify the following substances (2mks)
a. Solid A ………………………………………………………………………………………………
b. Gas D. ………………………………………………………………………………………………
c. Solid Q. ………………………………………………………………………………………………
d. Solution M. ………………………………………………………………………………………………
(ii) Write a chemical equation for the reaction in step I. (1mk)
(iii) Write chemical equation for the formation of the following compound.(3mks)
a. Solid G. ………………………………………………………………………………………………
b. Gas D. ………………………………………………………………………………………………
c. Light blue solution C. ………………………………………………………………………………………………
(iv) State the confirmatory test for oxygen gas. (1mk)
(v) Write the ionic equation for reaction taking place in process P. (1mk)
(vi) State one use of oxygen. (1mk)
9 marks