KCSE Chemistry Paper 3 – 2015 KCSE Ikutha Sub-County Joint Examination
2015 KCSE Ikutha Sub-County Joint Examination
Chemistry Paper 3
You are provided with:
– Solid V
– 2.0M hydrochloric acid, solution B
– 0.1M sodium hydroxide, solution C
You are required to determine the enthalpy change ∆H, for the reaction between solid V and one mole
of hydrochloric acid.
Procedure I
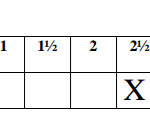
Using a burette, place 20.0cm3 of 2.0M hydrochloric acid, solution B in a 100ml beaker. Measure the
temperature of the solution after every half – minute and record the values in table 1. At exactly 2½
minutes, add all of solid V to the acid. Stir the mixture gently with a thermometer of mixture after every half minute and record the values in table I (Retain the mixture for use in procedure II)
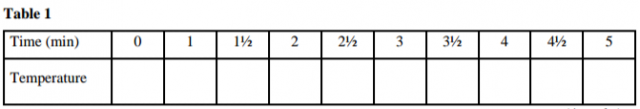
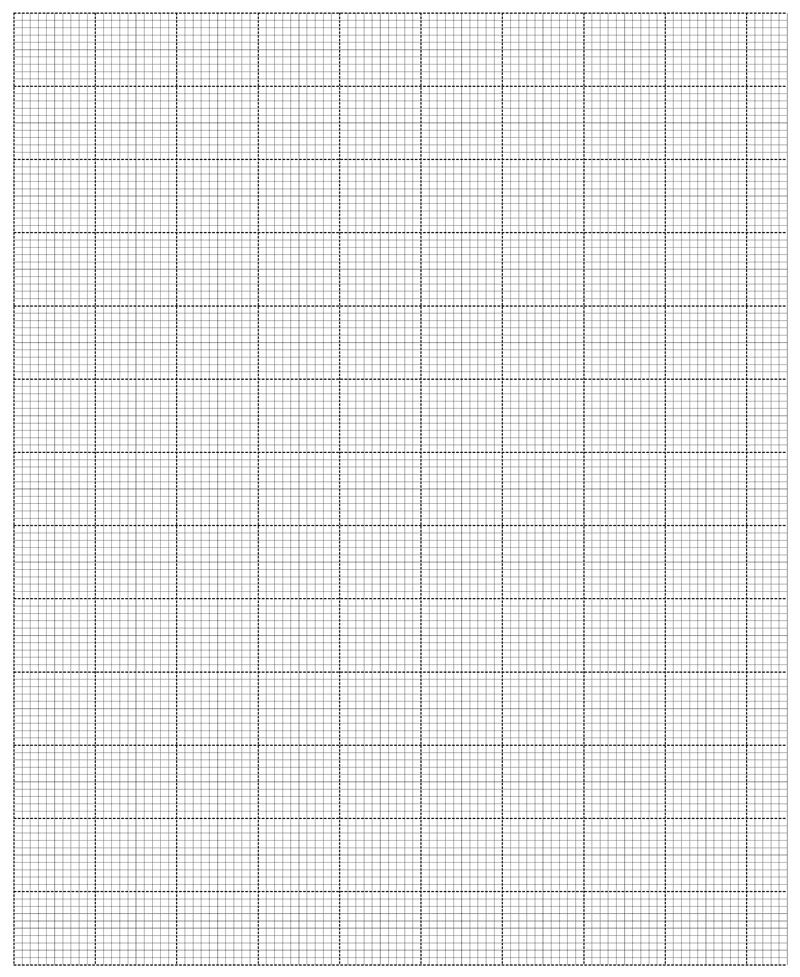
a) On the grid provided plot a graph of temperature (vertical axis) against time. (3 marks)
b) From the graph determine the change in temperature ∆T. (1 mark)
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
c) Calculate the heat change for the reaction (Assume that the specific heat capacity of the mixture is
4.2j/g/K and the density of the mixture is 1g/cm3
) (1 mark)
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
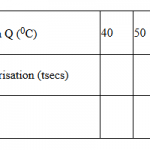
Procedure II
Rinse the burette thoroughly and fill it with 0.1M sodium hydroxide, solution C. Transfer all the
contents of the 100ml beaker used in procedure I into a 250ml volumetric flask. Add distilled water to
make up to the mark. Label this solution V. Using a pipette and a pipette filler, pipette 25.0cm3
of
solution V into a conical flask. Add 2 – 3 drops of phenolphthalein indicator and titrate with solution C.
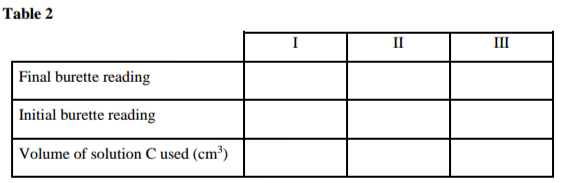
Record your results in table 2 below. Repeat titration two more times and complete table 2.
(4 marks)
Calculate the;
a) Average volume of sodium hydroxide used. (1 mark)
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
b) The number of moles of;
I. Sodium hydroxide used. (1 mark)
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
II) Hydrochloric acid in 25cm3
of solution V. (1 mark)
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
III) Hydrochloric acid in 250cm3 Solution V. (1 mark)
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
IV) Hydrochloric acid in 20.0 cm3 of solution V. (1 mark)
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
V) Hydrochloric acid that reacted with solid V. (1 mark)
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
c) Calculate the enthalpy of reaction between solid V and one mole of hydrochloric acid. (2 marks)
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
20 marks
You are provided with mixture N. You are required to:-
i) Carry out tests on mixture N
ii) Identify any gases produced if any
iii) Record your observations and inferences accordingly.
Procedure
i) Place a spatulaful of mixture N in a test tube.
ii) Add 8cm3
of distilled water and shake well
iii) Filter and retain the residue
a) Divide the filtrate into four parts.
b) Add sodium hydroxide to the first portion drop wise while observing till in excess.
c) Add ammonia solution to the second portion of the filtrate drop wise, until in excess.

e) Add a few drops of potassium iodide solution to the fourth portion.
f) Remove the residue from the filter paper and place it in a test tube, add 5cm3 of dilute nitric (V) acid.
12 marks
You are provided with organic compound solid G. Carry out the following tests.
a) Place all of solid G in a boiling tube. Add about 20cm3 of distilled water and shake well. Divide the
mixture into 3 separate test tubes.
b) To the first portion of the mixture add a spatula full of sodium carbonate solid.
c) To the second portion of the mixture, add a few drops of universal indicator and test the pH
d) To the third portion of the mixture, add 2cm3 of ethanol followed by 2 drops of concentrated sulphuric (VI) acid.
7 marks