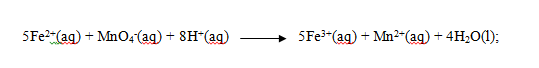KCSE Chemistry Paper 3 – Kabarak High School Mock 2015
Mock 2015 – Kabarak High School
Chemistry Paper 3
You are provided with:
- Solution P of Potassium manganate (VII).
- 0.05M solution Q of oxalic acid.
- Solution R containing 4.9g of ammonium iron (II) Sulphate, (NH4)2 SO4.FeSO4.6H2O, in 250cm3 of water.
You are required to:
i) Determine the rate of reaction between oxalic acid and Potassium manganate (VII).
ii) Standardize the solution P.
PROCEDURE I:
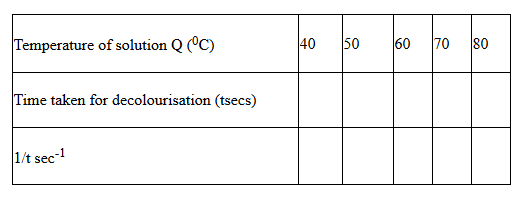
Using a measuring cylinder, place 1 cm3 of solution P into each of the five (5) test-tubes in a rack. Clean the measuring cylinder and use it to place 19 cm3 of solution Q into a boiling tube. Prepare a water bath by placing about 200 cm3 of water into a beaker and start to heat. Place a thermometer into solution Q and place it in the warm water until it attains a temperature of 400C. Remove the boiling tube from the water – bath and place it in the test-tube rack. Add the first portion of solution P immediately and at the same time start a stop watch. Record the time taken for solution P to be decolourised in table I below. Repeat the procedure at temperatures of 500C, 600C, 700C and 800C to complete the table.
i) Plot a graph of 1/t against temperature (X-axis). (3marks)
ii) From the graph determine the time taken for the mixture to decolourise at 650C (3marks)
iii) How does the rate of reaction between oxalic acid and Potassium manganate (VII) vary with temperature? (1mark)
PROCEDURE II
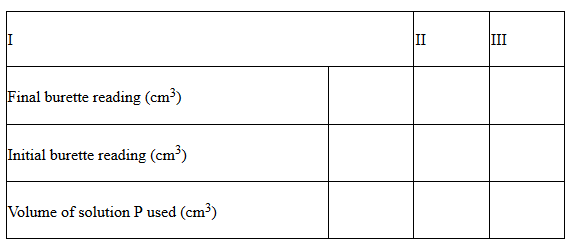
Fill a burette with solution P. Pipette 25cm3 of solution R into a conical flask and titrate the solution P against solution R until a permanent pink colour just appears. Record your results in table II below and repeat the procedure to fill the table.
Fill a burette with solution P. Pipette 25cm3 of solution R into a conical flask and titrate the solution P against solution R until a permanent pink colour just appears. Record your results in table II below and repeat the procedure to fill the table.
i) Determine the average volume of P used……………………..cm3 (1mark)
(Show how you arrive at your answer)
ii) Calculate the concentration of solution R in moles per litre. (Fe=56, S=32, O=16, N=14, H=1). (2marks)
iii) Find the number of moles of solution R used (1mark)
iv) Given the ionic equation for the reaction is
Find the number of moles of solution P used . (1mark)
v) Determine the concentration of the Potassium manganate (VII), solution P in moles per litre. (2 marks)
14 marks
You are provided with solid B. Carry out the tests below and record your observations and inferences in the table below.
i) Place half a Spaluta full of solid B in a clean dry test-tube and heat gently then strongly.
ii) Place the remaining solid B in a boiling tube and add about 5cm3 of distilled water and shake well. Divide the resulting mixture into four portions for the tests below.
a) To the first portion add Sodium hydroxide solution dropwise until in excess.
b) To the second portion add 2-3 drops of dilute Sulphuric (VI) acid
c) To the third portion add aqueous ammonia dropwise until in excess.
d) To the fourth portion add 2-3 drops of barium nitrate solution
12 marks
You are provided with solid L. Carry out the tests below on L and record the observations and inferences in the spaces provide.
a) Place half of solid L in a boiling tube and add about 5cm3 of distilled water. Divide the resulting mixture into two portions for the tests below:
i) To the first portion add 2-3 drops of acidified Potassium manganate (VII).[ Solution P]
ii) To the second portion dip a piece of blue litmus paper
b) Place the remaining solid L in a metallic spatula and ignite it.
6 marks