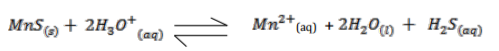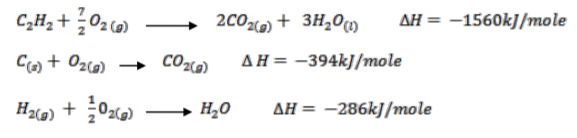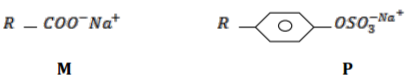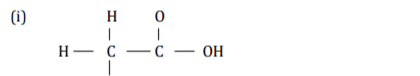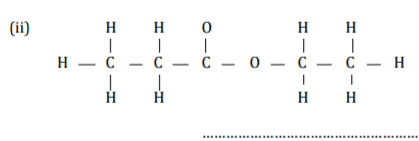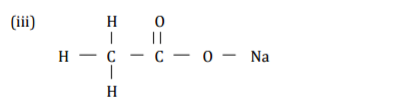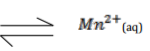KNEC Chemistry Paper 1 Question Paper / 2016 KCSE MOKASA Joint Examination
2016 KCSE MOKASA Joint Examination
Chemistry Paper 1
Explain the following:
(i) It is always advisable to scoop chemical substances using a clean spatula.
( ½ mark)
………………………………………………………………………………………………………………………….
(ii) Flammable substances should always be kept away from flames in the laboratory.
( ½ mark)
………………………………………………………………………………………………………………………….
1 marks
Name one reagent that can be used to distinguish between Al3+ and Zn2+ ions in solution
and state what would be observed if each of the ions is treated with the reagent you have
named.
……………………………………………………………………………………………………………………………………
……………………………………………………………………………………………………………………………………
………………………………………………………………………………………………………………………….…………
……………………………………………………………………………………………………………….……………………
…………………………………………………………………………………………………….………………………………
………………………………………………………………………………………………………………………….…………
……………………………………………………………………………………………………………….……………………
3 marks
Manganese sulphide reacts with acids according to the following equation.
State, giving a reason what would happen to the equilibrium if;
(i) Water is added to the equilibrium mixture. (1 ½ marks)
……………………………………………………………………………………………………………………………………
……………………………………………………………………………………………………………………………………
(ii) Hydrogen chloride is bubbled into the equilibrium mixture. (1 ½ marks)
……………………………………………………………………………………………………………………………………
……………………………………………………………………………………………………………………………………
……………………………………………………………………………………………………………………………………
3 marks
Use the thermochemical equations below to answer the questions that follow.
(i) Draw an energy cycle diagram to show the enthalpy of formation of ethane.
(1 ½ marks)
(ii) Calculate the enthalpy of formation of ethane. (1 ½ marks)
3 marks
State the conditions under which copper reacts with sulphuric acid and give an equation
for the reaction.
……………………………………………………………………………………………………………………………………
……………………………………………………………………………………………………………………………………
……………………………………………………………………………………………………………………………………
2 marks
When 8.8g of hydrocarbon Z was burnt in excess air, 14.4g of water and 11.95 dm3 of
carbon (IV) oxide were obtained at s.t.p. Determine the empirical formula of Z.
3 marks
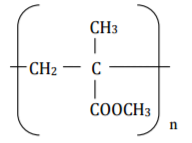
Perspex is a synthetic polymer of formula
(a) Write the structural formula of the monomer of Perspex. (1 mark)
(b) State the type of polymerization involved in the formation of perspex.
(1 mark)
……………………………………………………………………………………………………………………………………
2 marks
When zinc granules are dropped into two separate solutions of dilute sulphuric (VI) and
concentrated sulphuric (VI) acid, effervescence of a colourless gas occurs in each case.
Give equations to represent the reactions that take place.
……………………………………………………………………………………………………………………………………
……………………………………………………………………………………………………………………………………
2 marks
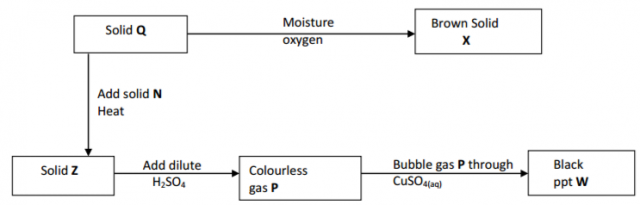
Study the chart below and answer the questions that follow.
(a) Identity solid X. (1 mark)
…………………………………………………………………………………………………………………………
(b) Write an ionic equation for the reaction between P and copper (II) sulphide
solution. (1 mark)
……………………………………………………………………………………………………………………………………
(c) State the observation made when gas P is bubbled through iron (III) chloride
solution. (1 mark)
……………………………………………………………………………………………………………………………………
3 marks
Use the nuclear equations below to answer the questions that follow.
(i)
(ii)
(a) Give the actual names of particles X and Y. (1 mark)
X ………………………………………………………………………………………………
Y ………………………………………………………………………………………………
(b) Give the name of a radiation whose emission does not change the mass number
or the atomic number of a radioisotope. (1 mark)
…………………………………………………………………………………………………………………………
2 marks
The structures below represent two cleaning agents M and P.
Which cleaning agent would be most suitable for use with water containing calcium
sulphate. Give a reason.
…………………………………………………………………………………………………………………………………….
…………………………………………………………………………………………………………………………………….
2 marks
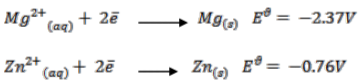
You are given the following half equations:
(i) Obtain an equation of the cell reaction. (1 mark)
…………………………………………………………………………………………………………………………………….
(ii) Calculate the 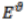
(iii) Give the oxidizing species. (1 mark)
…………………………………………………………………………………………………………………………………….
3 marks
Using dots (●) and crosses (×) to represent outermost electrons; draw diagrams to show
bonding in:
(a) Aluminium chloride. (1 ½ marks)
(b) Sulphuric (IV) oxide. (1 ½ marks)
3 marks
Use the information in the table below to answer the questions that follow.
| Melting point | Element | Atomic number |
| 97.8 | R | 11 |
| 660 | S | 13 |
| 1440 | T | 14 |
| -40.1 | U | 17 |
| 63.1 | V | 19 |
(a) Write the electron arrangement of: (1 mark)
(i) ion of S ……………………………………………….
(ii) atom of T ……………………………………………….
(b) Explain why the melting point of T is higher than that of U. (2 marks)
…………………………………………………………………………………………………………………………………….
…………………………………………………………………………………………………………………………………….
…………………………………………………………………………………………………………………………………….
…………………………………………………………………………………………………………………………………….
…………………………………………………………………………………………………………………………………….
3 marks
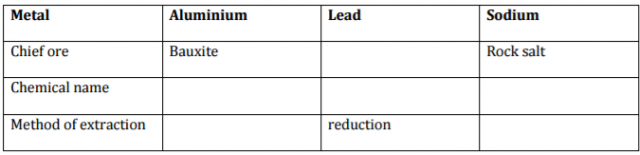
Complete the table below.
3 marks
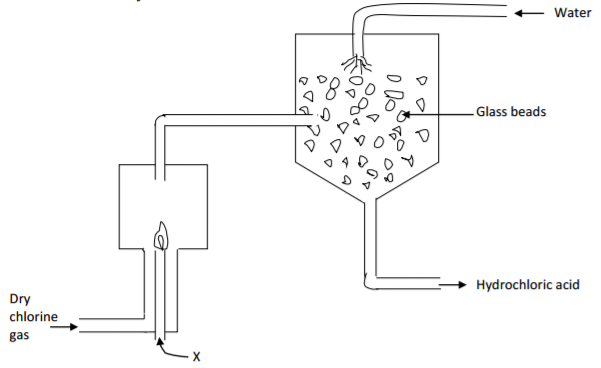
The diagram below represents a set up used for the large scale manufacture of hydrochloric acid.
(a) Name substance X. (1 mark)
…………………………………………………………………………………………………………………………………….
(b) What is the purpose of the glass beads? (1 mark)
…………………………………………………………………………………………………………………………………….
…………………………………………………………………………………………………………………………………….
(c) Give one use of hydrochloric acid. (1 mark)
…………………………………………………………………………………………………………………………………….
…………………………………………………………………………………………………………………………………….
3 marks
Calculate the volume of nitrogen (I) oxide produced when 38.2g of ammonium nitrate is
completely decomposed by heating (at s.t.p). (N = 14, H = 1, O = 16)
3 marks
Give equations to show the reactions that take place when;
(a) iron reacts with steam. (1 mark)
…………………………………………………………………………………………………………………………………….
(b) Give one industrial use of the gas produced in the reactions in (i) and (ii) above.
(1 mark)
…………………………………………………………………………………………………………………………………….
…………………………………………………………………………………………………………………………………….
2 marks
(a) When magnesium metal is burnt in air, it reacts with both oxygen and nitrogen
gases giving a white ash. Write two equations for the reactions that take place.
(2 marks)
…………………………………………………………………………………………………………………………………….
…………………………………………………………………………………………………………………………………….
(b) Give the total number of atoms present in the gas produced when water is added
to magnesium nitrate. (1 mark)
………………………………………………………………………………………………………………………….
3 marks
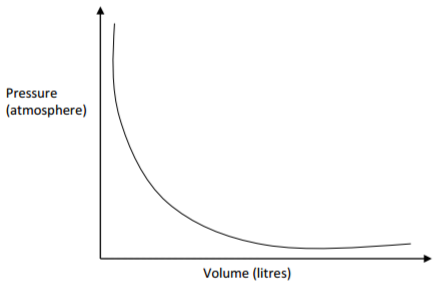
The graph below shows the behavior of a fixed mass of a gas at constant temperature.
(a) What is the relationship between the volume and the pressure of the gas?
(1 mark)
………………………………………………………………………………………………………………………….
………………………………………………………………………………………………………………………….
………………………………………………………………………………………………………………………….
(b) 60 cm3 of oxygen gas diffused through a porous partition in 50 seconds. How
long would it take 60cm3 of sulphur (IV) oxide gas to diffuse through the same
partition under the same conditions? (S = 32.), O = 16.0)
(3 marks)
2 marks
State and explain the observation made when a moist red litmus paper is put in a gas jar
of dry chlorine gas.
…………………………………………………………………………………………………………………………………..
…………………………………………………………………………………………………………………………………..
…………………………………………………………………………………………………………………………………..
2 marks
(a) When extinguishing a fire caused by burning kerosene, charbon (IV) oxide is
preferred to water. Explain. (2 marks)
…………………………………………………………………………………………………………………………………..
…………………………………………………………………………………………………………………………………..
…………………………………………………………………………………………………………………………………..
(b) Write the formula of the oxide of carbon which is ‘silent killer’. (1 mark)
…………………………………………………………………………………………………………………………………..
3 marks
Explain why chlorine is a gas while iodine is a solid at room temperature.
…………………………………………………………………………………………………………………………………..
…………………………………………………………………………………………………………………………………..
…………………………………………………………………………………………………………………………………..
…………………………………………………………………………………………………………………………………..
2 marks
Apart from their location, state any two differences between a proton and an electron.
…………………………………………………………………………………………………………………………………..
…………………………………………………………………………………………………………………………………..
…………………………………………………………………………………………………………………………………..
…………………………………………………………………………………………………………………………………..
2 marks
What term is given to: The amount of energy given out when a neutral atom in gaseous
state gains an electron?
…………………………………………………………………………………………………………………………………..
1 marks
A certain fertilizer is suspected to be containing nitrate ions. Describe how the presence
of nitrate ions can be determined in such fertilizer.
…………………………………………………………………………………………………………………………………..
…………………………………………………………………………………………………………………………………..
…………………………………………………………………………………………………………………………………..
…………………………………………………………………………………………………………………………………..
…………………………………………………………………………………………………………………………………..
…………………………………………………………………………………………………………………………………..
…………………………………………………………………………………………………………………………………..
…………………………………………………………………………………………………………………………………..
…………………………………………………………………………………………………………………………………..
3 marks
Write balanced chemical equations to show the action of heat on the following nitrates.
(a) Lead (II) nitrate (1 mark)
…………………………………………………………………………………………………………………………………..
(b) Silver nitrate (1 mark)
…………………………………………………………………………………………………………………………………..
2 marks
Starting with zinc carbonate solid describe how zinc hydroxide can be prepared in the
laboratory.
…………………………………………………………………………………………………………………………………..
…………………………………………………………………………………………………………………………………..
…………………………………………………………………………………………………………………………………..
3 marks
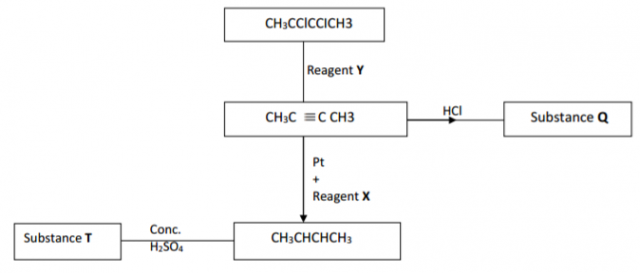
Below is a scheme of some reactions starting with but-z-yne. Study it and answer the
questions that follow.
(a) Name Y, X and T (1 ½ marks)
Y ……………………………………………………………………………….
X ……………………………………………………………………………….
T ……………………………………………………………………………….
(b) Give the name of the following organic compounds. ( ½ mark)
2 marks
The following results were obtained during an experiment to determine the solubility of
potassium nitrate in water at 300C. Mass of dish = 15.86g, mass of dish + saturated
solution at 30oC = 26.86g, mass of dish + solid KNO3 after evaporation to dryness = 16.7g. Calculate the mass of saturated solution containing 60.0g of water at 30oC.
2 marks