KNEC KCSE Chemistry Paper 1 Question Paper / 2015 KCSE Starehe Boys Centre Mock
2015 KCSE Starehe Boys Centre Mock
Chemistry Paper 1
The two different flames produced by a Bunsen burner were separately used to heat 100cm3 of water in 250cm3 beaker. The water heated using flame A took 13 minutes to boil while the water heated using flame B took 9minutes and 25 seconds to boil.
Identify flame A and draw a labeled diagram of the flame, showing all its regions.
3 marks
Name
(i) the most abundant gas found in air; (1mark)
(ii) Two gases found in air that causes iron to rust. (1mark)
(iii) The most abundant noble gas found in air. (1mark)
3 marks
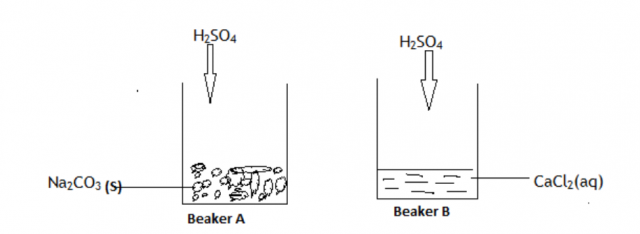
Sodium nitrate crystals were mixed with lead (II) chloride salt. Explain briefly how you can separate the crystals of sodium nitrate from this mixture.
3 marks
Element A burns with a blue flame in air forming a colourless gas B. The gas formed turns wet blue litmus red and after sometime, the litmus turns white.
(a)Name element A and gas B. (1mark)
(b)Give the nature of gas B. (1mark)
(c)Write an equation for the reaction that caused red litmus to turn white. (1mark)
3 marks
What colour would blue cobalt (II) chloride paper turn on exposure to air for some time. Explain.
2 marks
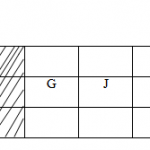
Below is a table of some particles (not their actual chemical symbols) showing the number of protons, neutron and electrons.
| Particle | Protons | Neutrons | Electrons |
| K | 12 | 12 | 10 |
| L | 17 | 18 | 17 |
| M | 7 | 7 | 10 |
| N | 17 | 20 | 18 |
| Q | 10 | 10 | 10 |
(a)Choose;
(i)A cation. (½mark)
(ii)Neutral atom of a non metal. (½mark)
(iii)A pair of isotopes. (½mark)
(b)Using crosses(x) and dots (.) draw the structure of particle M. (1½ mark)
3 marks
Argon has three isotopes which are argon-36, argon-38 and argon-40. Determine the percentage composition of argon-40 given that the relative atomic mass of argon is 39.9852 and argon-36 has percentage abundance of 0.34%.
3 marks
Elements X and Y are in period 3 of the periodic table. The chemical formula of their chlorides is XCl2 and YCl4 respectively. The chloride of X dissolve in water producing a solution with a pH of 7 while the chloride of Y dissolve in water producing a solution with a pH of 3.
Determine the type of bond and structure of the chlorides of X and Y. (X and Y are not chemical symbols of an element. Chlorine is a halogen).
(2marks)
Draw a cross(x) dot (.) diagram of the chloride of Y. (1mark)
3 marks
A molten oxide of metal M (not the actual chemical symbol of the element) was electrolyzed using graphite. The chemical formula of the metal oxide is M2O3.
(i)The solid metal oxide does not conduct electricity but only conduct in liquid state. Explain. (1mark)
(ii)Write half equations for the reactions that took place at the;
(a)Anode. (1mark)
(b)Cathode. (1mark)
3 marks
A pellet of sodium hydroxide left exposed to air underwent the following changes:
(i)Changed into a colourless liquid, then
(ii)Formed colourless transparent crystals, and finally
(iii)The crystals formed a white powder.
(a)Use one word to describe each of the changes in (i) and (iii).
(i) (1mark)
(iii) (1mark)
(b)Write an equation for change (ii). (1mark)
3 marks
When a current of 0.5 amperes was passed through the fused chloride of metal Z (ZCl2) for 20 minutes and 20 seconds, 0.278 g of Z were deposited at the cathode. Determine the relative atomic mass of Z. (1 Faraday =96500C).
3 marks
(i)What is meant by the term cracking of alkanes. (1mark)
(ii) Cracking of heptane gives propene and another hydrocarbon Y as the only products. Draw and name two isomers of Y. (2marks)
3 marks
Aluminium hydroxide reacts with acid and alkalis.
(a)Write an equation for the reaction between aluminium hydroxide and:
(i)Dilute hydrochloric acid. (1mark)
(ii)Potassium hydroxide. (1mark)
(b)What property of aluminium hydroxide is shown by the reactions in (a) above. (1mark)
3 marks
(a) Write the chemical formula of the compounds that causes temporary water hardness. (1marks)
(b) Write equations for reaction that take place when temporary hardness is removed by addition of ammonia solution. (2marks)
3 marks
(i) What is a 0.5molar nitric (V) acid solution? (1mark)
(ii) Calculate the volume of water that must be added to 20cm3 of 4M nitric (V) acid solution to make a 0.5M solution. (2marks)
3 marks
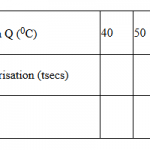
Study the table below showing solubility of a salt at various temperatures.
| Temperature (C) | Solubility (g/100g water) |
| 0 | 30 |
| 30 | 24 |
| 70 | 19 |
| 100 | 14 |
325g of saturated solution at 00C was heated to a temperature of 1000 C. calculate the mass of salt crystallized out.
3 marks
Silver nitrate solution was electrolyzed using graphite cathode and silver anode for some time.
(a)State the observation made at anode. (1mark)
(b)Explain the effect of this electrolysis on the PH of the solution. (1mark)
(c)Write an equation for the reaction that took place at the anode.(1mark)
3 marks
(a)What is half life of a radioactive element? (1mark)
(b)224 grams of a radioactive element W disintegrate to 7grams in 100days. Determine the half life of the element W. (2marks)
3 marks
State three properties of carbon (IV) oxide that makes it suitable for use in fire extinguishers.
3 marks
Study the equilibrium reaction below and answer the questions that follow.
The forward reaction is exothermic. How would the following affect the position of the equilibrium?
(a) The temperature of the system is lowered. Explain. (1½ mark)
(b)The pressure of the system is lowered. Explain. (1½ mark)
3 marks
The molar heat of combustion of methane is -890kJ/mole. Calculate the mass of methane that is burnt to cause the temperature of 500cm3 of water to rise from 21.00C to 36.00C.(Take the specific heat capacity of water to be 4.2kJ kg-1K-1, density of water is 1g/cm3and C=12,H=1)
3 marks
When potassium manganate(VII) is heated strongly, the solid changes its colour from purple to form a residue of green and black solids and a colourless gas Y.
(a)Write an equation for the reaction that took place. (1mark)
(b)Describe the test for gas Y. (1mark)
(c)Gas Y is collected over water. Explain. (1mark)
3 marks
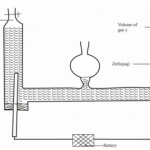
Draw a labeled diagram of set up of apparatus that can be used to prepare a dry sample of hydrogen gas when hydrochloric acid is reacted with zinc metal.
3 marks