KNEC KCSE Chemistry Paper 1 Question Paper / 2016 KASSU JET JOINT EXAMINATION
2016 KASSU JET JOINT EXAMINATION
Chemistry Paper 1
(a) Give two differences between luminous and non-luminous flames.(2 marks)
………………………………………………………………………………………………………………………..
………………………………………………………………………………………………………………………..
………………………………………………………………………………………………………………………..
(b) How is the non-luminous flame produced? (1 mark)
………………………………………………………………………………………………………………………..
………………………………………………………………………………………………………………………..
3 marks
(a) The apparatus below were used to separate a mixture of liquid A and B.
State two properties of liquids that make it possible to separate using such
apparatus. (2 marks)
………………………………………………………………………………………………………………………..
………………………………………………………………………………………………………………………..
(b) Give the name of the above apparatus. (1 mark)
………………………………………………………………………………………………………………………..
3 marks
(a) Explain why solid Carbon (IV) oxide is preferred over ordinary ice for use by ice
cream venders. (1 mark)
………………………………………………………………………………………………………………………..
………………………………………………………………………………………………………………………..
(b) Name one piece apparatus used to measure volume of gases. (1 mark)
………………………………………………………………………………………………………………………..
(c) Draw a diagram of a deflagrating spoon. (1 mark)
3 marks
The table below shows the pH values of solutions P, R, Q and S.
| Solution | P | R | Q | S |
| pH | 2 | 7 | 6.5 | 13.5 |
(a) Which solution represent:
(i) Strong base – ……………………………………………… (1 mark)
(ii) Weak acid – ………………………………………………. (1 mark)
(b) Give an example of solution S. (1 mark)
………………………………………………………………………………………………………………………..
3 marks
6.95g of hydrated iron (II) sulphate FeSO4. nH2O was dissolved in 250 cm3 solution
resulting into a 0.1M solution. Determine the value of n.
(Fe = 56, O = 16, S = 32, H = 1).
3 marks
Rusting leads to fast wearing out of farm tools and equipment as well as buildings.
(a) Give the chemical name of rust. (1 mark)
………………………………………………………………………………………………………………………..
(b) What two conditions accelerate rusting process? (2 marks)
………………………………………………………………………………………………………………………..
………………………………………………………………………………………………………………………..
3 marks
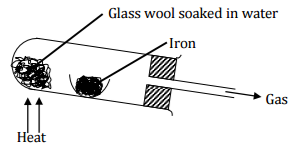
Study the diagram below and answer the questions that follow.
(a) Write an equation for the reaction that take place in the combustion tube.
(1 mark)
………………………………………………………………………………………………………………………..
(b) Why would it not be advisable to use potassium in place of iron in the set-up?
(1 mark)
………………………………………………………………………………………………………………………..
………………………………………………………………………………………………………………………..
(c) Glass wool should be heated before heating iron. Explain. (1 mark)
………………………………………………………………………………………………………………………..
………………………………………………………………………………………………………………………..
3 marks
I. Name the following organic compounds.
(a)
……………………………………………………………………………………………………………..
(b) HOCH2– CHOH – CH2OH (1 mark)
……………………………………………………………………………………………………………..
II. Given
A CH3(CH2)16 COO– Na+
B CH3(CH2)6 CH(CH3)CH2 SO-3 Na+
Identify detergent
A – ………………………………………………………………………. (1 mark)
B – ………………………………………………………………………. (1 mark)
4 marks
In terms of structure and bonding, explain the following.
(a) Graphite is used as a lubricant. (1 mark)
………………………………………………………………………………………………………………………..
………………………………………………………………………………………………………………………..
………………………………………………………………………………………………………………………..
(b) Alluminium is better conductor of electricity then magnesium. (1 mark)
………………………………………………………………………………………………………………………..
………………………………………………………………………………………………………………………..
(c) Water is a liquid at room temperature while hydrogen sulphide is a gas.
(1 mark)
………………………………………………………………………………………………………………………..
………………………………………………………………………………………………………………………..
3 marks
(a) Define the term molar latent heat of fusion. (1 mark)
………………………………………………………………………………………………………………………..
………………………………………………………………………………………………………………………..
(b) The molar heat of fusion of ice at O0C is 6kJ mol-1. Calculate the heat change
when 36g of ice is converted to 36g of water at 100C. (3 marks)
(SHC = 4.2-1g K-1, density = 1.0g/cm33, H = 1.0, O = 16.0)
4 marks
Draw a well labeled diagram showing how blister copper is purified.
3 marks
Gas Q with a relative molecular mass of 48 took 50 seconds to diffuse through a porous
diaphragm. How long will it take for the same amount of hydrogen Chloride (HCl) to
diffuse through the same diaphragm under similar conditions? (H = 1.0, Cl = 35.5).
3 marks
(a) Calculate the oxidation state of chromium in the ion Cr2 O2–. (1 mark)
(b) Using oxidation numbers, determine from the equation below the species which
undergoes oxidation and reduction.
2FeCl2(aq) + Cl2 (g) 2Fe Cl3 (aq)
Oxidation – ……………………………………………………………… (1 mark)
Reduction – ……………………………………………………………… (1 mark)
3 marks
Given elements A, B and C with atomic numbers 11, 19 and 13 respectively.
(a) Compare the atomic radius of A and C. Explain. (2 marks)
………………………………………………………………………………………………………………………..
………………………………………………………………………………………………………………………..
(b) Compare reactivity of A and B. (1 mark)
………………………………………………………………………………………………………………………..
3 marks
Haber process (the manufacture of ammonia gas) is given by the following equation.
N2 (g) + 3H2 (g) 2NH3 (g) ΔH = -92kJ mol-1.
State and explain the effect of:
(a) Introducing some drops of water to the equilibrium. (1 mark)
………………………………………………………………………………………………………………………..
………………………………………………………………………………………………………………………..
(b) Pumping nitrogen gas to the equilibrium mixture. (1 mark)
………………………………………………………………………………………………………………………..
………………………………………………………………………………………………………………………..
(c) Lowering the temperature of the reaction. (1 mark)
………………………………………………………………………………………………………………………..
………………………………………………………………………………………………………………………..
3 marks
Elements P and Q have the following atomic numbers 19 and 8 respectively.
(i) Using dot ( ) and cross draw a diagram to show how the elements form bonds.
(1 mark)
1 marks
Describe how sodium sulphate crystals can be prepared starting with 50cm3 of 2M
sodium hydroxide and 1M sulphuric (VI) acid.
…………………………………………………………………………………………………………………………………..
…………………………………………………………………………………………………………………………………..
…………………………………………………………………………………………………………………………………..
…………………………………………………………………………………………………………………………………..
…………………………………………………………………………………………………………………………………..
3 marks
Write ionic equations to show how;
(a) (i) Excess ammonia solution reacts with a solution containing Copper II ions. (1 mark)
…………………………………………………………………………………………………………..
(ii) Excess sodium hydroxide added to a solution containing Al3+ ions.
(1 mark)
…………………………………………………………………………………………………………..
(b) Give the name of the following ion [Zn(NH3)4]2+ (1 mark)
………………………………………………………………………………………………………………………..
3 marks
(a) Define electrolysis. (1 mark)
………………………………………………………………………………………………………………………..
………………………………………………………………………………………………………………………..
(b) During the electrolysis of molten aluminium oxide, write the equations at the;
Anode – …………………………………………………………………………. (1 mark)
Cathode – …………………………………………………………………………. (1 mark)
3 marks
(a) Give any two differences between alpha and beta particles. (2 marks)
………………………………………………………………………………………………………………………..
………………………………………………………………………………………………………………………..
(b) A radioactive isotope T decays by emitting three alpha particles to form 21483 Bi
what is the atomic number and mass number T?
Atomic number – …………………………………………………… (1 mark)
Mass number – …………………………………………………… (1 mark)
4 marks
(a) Using acidified potassium dichromate (VI) solution, describe how you would
differentiate between sulphur (IV) oxide and hydrogen sulphide. (2 marks)
………………………………………………………………………………………………………………………..
………………………………………………………………………………………………………………………..
………………………………………………………………………………………………………………………..
(b) Identify the catalyst preffered in contact process. Explain. (2 mark)
………………………………………………………………………………………………………………………..
………………………………………………………………………………………………………………………..
………………………………………………………………………………………………………………………..
4 marks
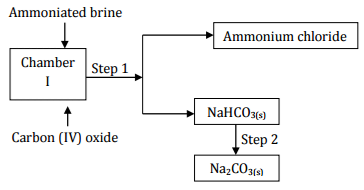
Study the following part of the solvay process for the manufacture of sodium carbonate
and answer the questions that follows:
(i) State the main source of Carbon (IV) oxide in the process. (1 mark)
………………………………………………………………………………………………………………………..
(ii) Write down the overall equation for the reaction in chamber I. (1 mark)
………………………………………………………………………………………………………………………..
(iii) Name process in step 1. (1 mark)
………………………………………………………………………………………………………………………..
3 marks
(a) The following equation involve hydrochloric acid.
MnO2 (s) + 4HCl(aq) MnCl2 (aq) + 2H2O(l) + Cl2 (g)
State the type of reaction taking place in the reaction. (1 mark)
………………………………………………………………………………………………………………………..
(b) State two contrasting chemical properties of hydrogen and chlorine.
(2 marks)
………………………………………………………………………………………………………………………..
………………………………………………………………………………………………………………………..
………………………………………………………………………………………………………………………..
3 marks
(a) An element O has two isotopes 168 O containing 90% and Isotope 188 O.
(i) What are isotopes? (1 mark)
……………………………………………………………………………………………………………..
……………………………………………………………………………………………………………..
(ii) Find the R.A.M of O. (2 marks)
3 marks
(a) When a hydrocarbon is completely burnt in oxygen 4.2g of Carbon (IV) oxide and 1.71g of water were formed.
(a) Determine the empirical formular of the hydrocarbon. (3 marks)
(b) Given that formula mass of compound above is 28. Find the molecular
formular. (1 mark)
4 marks
(a) Name the two types of polymerization. (1 mark)
………………………………………………………………………………………………………………………..
………………………………………………………………………………………………………………………..
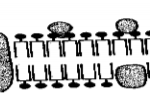
(b) Study the section of the polymer below and answer the questions that follow.
(i) Give the name of the polymer above. (1 mark)
……………………………………………………………………………………………………………..
2 marks