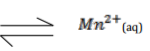KNEC KCSE Chemistry Paper 1 Question Paper / 2016 KCSE 4MCK Joint Exam
2016 KCSE 4MCK Joint Exam
Chemistry Paper 1
a) Explain why an atom is electrically neutral. (1 mark)
………………………………………………………………………………………………
………………………………………………………………………………………………
b) An ion is represented as P3- and the electron arranged is 2⋅8⋅18⋅8. Write the
electron arrangement of the atom. (1 mark)
………………………………………………………………………………………………
………………………………………………………………………………………………
2 marks
a) What is an ion. (1 mark)
………………………………………………………………………………………………
………………………………………………………………………………………………
b) Using dots (•) and crosses (x) to represent electrons draw a diagram of the
compound formed between Lithium and Oxygen (Li = 3, O = 8). (2 marks)
3 marks
Study the table below and answer the question that follow. (The letters are not the
actual symbols of elements).
| Element (Metals) |
Ionic radius |
First ionization energy (kJ/Mol) |
Second ionization energy (kJ/Mol) |
| P | 0.031 | 900 | 1800 |
| R | 0.065 | 736 | 1450 |
| Q | 0.099 | 590 | 1150 |
i) Which element is the most reactive? Explain. (2 marks)
…………………………………………………………………………………………………
………………………………………………………………………………………………….
…………………………………………………………………………………………………
………………………………………………………………………………………………….
ii) Give a reason as to why the 2nd ionization energy for element R is higher than its 1st
ionization energy. (1 mark)
…………………………………………………………………………………………………
…………………………………………………………………………………………………..
3 marks
The diagram below is a chromatogram of different pigments of a leaf extract.
Y
a) Name a suitable solvent for the dye. (1 mark)
………………………………………………………………………………………………………
………………………………………………………………………………………………………
b) Giving a reason, state the least soluble pigment. (1 mark)
………………………………………………………………………………………………………
………………………………………………………………………………………………………
c) On the diagram show the direction of movement of the solvent. (1 mark)
3 marks
If it takes 30 seconds for 100cm3 carbon (iv) oxide to diffuse across a porous pot.
How long will it take for equal volume of sulphur (VI) oxide (C = 12, O = 16, S = 32)
to dispose through the same porous pot?
………………………………………………………………………………………………………
………………………………………………………………………………………………………
………………………………………………………………………………………………………
………………………………………………………………………………………………………
3 marks
Study the flow chart below and answer the questions that follow:
a) Identify the following
i) Solid T (1 mark)
………………………………………………………………………………………………
ii) Gas L (1 mark)
………………………………………………………………………………………………
b) Write an equation for the reaction between Solid T and dilute Nitric (v) acid.
(1 mark)
………………………………………………………………………………………………
………………………………………………………………………………………………
3 marks
a) State two differences between nuclear and chemical reactions. (2 marks)
………………………………………………………………………………………………………
………………………………………………………………………………………………………
………………………………………………………………………………………………………
………………………………………………………………………………………………………
b) Below is a radioactive decay series. Study it to answer questions that follow.
𝐵𝐵𝐵𝐵 83
214 step I 𝑇𝑇𝑇𝑇 81
210 step II 82𝑃𝑃𝑃𝑃 210 step III 𝐵𝐵𝐵𝐵 83
210 step IV 84𝑃𝑃𝑃𝑃 210 step V 82𝑃𝑃𝑃𝑃 206
Identify the particles emitted in step III and step V.
Step III ……………………………………………………………………… (1 mark)
Step V………………………………………………………………………………(1 mark)
4 marks
The set up below was used by students from a secondary school to study reaction of
alkaline earth metals. Magnesium
Steam Point P
Apparatus C
Heat
a) Why is it important to burn the gas at point P. (1 mark)
…………………………………………………………………………………………………
…………………………………………………………………………………………………
b) Write the equation for the reaction taking place in the combustion tube. (1 mark)
…………………………………………………………………………………………………
………………………………………………………………………………………………….
c) Before heating the Magnesium, steam is first passed through the apparatus C. Explain.
(1 mark)
…………………………………………………………………………………………………
…………………………………………………………………………………………………
4 marks
Study the information in the table below and answer question below the table.
| Bond H – Cl Cl – Cl C – H C – Cl |
Bond energy kj/mol 431 244 414 326 |
Calculate the enthalpy change for the reaction,
CH4 + Cl2 CH3Cl + HCl
………………………………………………………………………………………………………
………………………………………………………………………………………………………
3 marks
R-coo– Na+ and R-C6H5SO3–Na+ represent two cleaning agents where R is a long
hydrogen chain.
a) Which cleaning agent will be suitable in water with calcium ions. (1 mark)
………………………………………………………………………………………………………
………………………………………………………………………………………………………
b) Write formulae of the compound formed between cleaning agent in (a) above and
calcium ions. (1 mark)
………………………………………………………………………………………………………
………………………………………………………………………………………………………
c) Which of the two cleaning agents does not pollute environment. (1 mark)
………………………………………………………………………………………………………
………………………………………………………………………………………………………
3 marks
The table below gives three experiments on the reaction of excess sulphuric acid and
0⋅5g of Zinc done under different conditions. In each case the volume of gas produced
was recorded at different time intervals.
| Experiment | Form of Zinc | Sulphuric acid concentration |
| A | Powder | 0.8M |
| B | Powder | 1.0M |
| C | Granules | 0.8M |
On the axis below, sketch and label the three curves that could be obtained from such results.
3 marks
The diagram below shows a Bunsen burner in use.
a) Name the regions B and C. (2 marks)
………………………………………………………………………………………………………
………………………………………………………………………………………………………
………………………………………………………………………………………………………
……………………………………………………………………………………………
b) Which region has partially burnt gases. (1 mark)
…………………………………………………………………………………………………
……………………………………………………………………………………………
3 marks
The diagram below is an incomplete set up used to prepare and collect Hydrogen
sulphide gas. S.
a. Complete the set up to show how the dry gas is collected. (2 marks)
b. Identify solid M. (1 mark)
…………………………………………………………………………………………
c. Describe a chemical test for the gas. (1 mark)
…………………………………………………………………………………………
4 marks
Starting with lead (II) Oxide, describe how crystals of lead (II) Nitrate can be
prepared.
………………………………………………………………………………………………
………………………………………………………………………………………………
………………………………………………………………………………………………
………………………………………………………………………………………………
………………………………………………………………………………………………
3 marks
a) What is positional isomerism. (1 mark)
………………………………………………………………………………………………
………………………………………………………………………………………………
c) Draw and name two isomers of Butene. (2 marks)
3 marks
a) State Gay lussac’s law. (1 mark)
………………………………………………………………………………………………
………………………………………………………………………………………………
b) 30cm3 of propane was mixed with 100cm3 of oxygen and mixture sparked. Calculate and
identify residual gases when measured at room temperature and pressure. (3 marks)
………………………………………………………………………………………………………
………………………………………………………………………………………………………
4 marks
You are given the following half equations.
I2(s) + 2e′ 2I-(aq) E∅ = + 0⋅54V
Br2(I) + 2e′ 2Br′(aq) E∅ = + 1⋅09V
a) Write an overall equation for the cell reaction. (1 mark)
…………………………………………………………………………………………………
…………………………………………………………………………………………………
b) Calculate the E∅ value of the cell. (1 mark)
…………………………………………………………………………………………………
…………………………………………………………………………………………………
c) Name the reading species. (1 mark)
…………………………………………………………………………………………………
…………………………………………………………………………………………………
4 marks
The diagram below was used by a student to prevent rusting of an iron spatula.
Zinc rod
Iron spatula Tap water
a) Did the student succeed in preventing rusting of the spatula? Explain. (2 marks)
…………………………………………………………………………………………………
…………………………………………………………………………………………………
b) Apart from the method being investigated above, mention two more methods of
preventing rusting. (2 marks)
…………………………………………………………………………………………………
…………………………………………………………………………………………………
4 marks
Calculate the number of hydrogen ions in 5cm3 of 0⋅5 M Phosphoric acid. (L = 6⋅0 x 1023)
………………………………………………………………………………………………………
………………………………………………………………………………………………………
………………………………………………………………………………………………………
………………………………………………………………………………………………………
3 marks
The following table shows the Ph values of solutions A,B,C and D.
| Solution | A | B | C | D |
| pH | 7 | 3 | 14 | 10 |
a) Which solution is likely to be that of iron (III) Chloride. Explain. (2 marks)
………………………………………………………………………………………………………
………………………………………………………………………………………………………
………………………………………………………………………………………………………
………………………………………………………………………………………………………
b) Which two solutions when mixed will give the highest enthalpy change (1 mark)
………………………………………………………………………………………………………
………………………………………………………………………………………………………
3 marks
Study the arrangement below and answer the question that follow;
Burning Candle
Calcium Hydroxide
Bench
Explain what will be observed after sometime.
………………………………………………………………………………………………………
………………………………………………………………………………………………………
………………………………………………………………………………………………………
………………………………………………………………………………………………………
3 marks
A piece of burning magnesium is lowered in a gas jar full of carbon (IV) Oxide.
a) Although carbon (IV) oxide gas does not support burning, the magnesium
continues burning, explain this observation. (1 mark)
………………………………………………………………………………………………………
………………………………………………………………………………………………………
b) Write the equation for the reaction. (1 mark)
………………………………………………………………………………………………………
………………………………………………………………………………………………………
c) State one physical property why CO2 gas is used in fire extinguishers. (1 mark)
………………………………………………………………………………………………………
………………………………………………………………………………………………………
3 marks
Metal H can displace oxygen from oxide of G, but not of J. Metal K removes oxygen
from Oxide J and also oxide H. Arrange the metals in order of increasing reactivity.
………………………………………………………………………………………………………
………………………………………………………………………………………………………
2 marks
Complete the diagram below to show how the Radioactive particles are stopped.
Lead
Uranium Lead block
Aluminium foil
Sheet of paper
3 marks
The following equation shows a reversible reaction.
H2(g) + Br 2(g) 2HBr(g) DH = -74⋅.4kj
Brown Colorless
a) State and explain the observation that can be made when
i. Temperature is increased. (1½ marks)
………………………………………………………………………………………………………
………………………………………………………………………………………………………
………………………………………………………………………………………………………
iii) Pressure is reduced (1½ marks)
………………………………………………………………………………………………………
………………………………………………………………………………………………………
………………………………………………………………………………………………………
3 marks
The solubility of salt X is 80g/100g of water at a temperature of 90°c. Solution is
cooled to 40°c.
a) Explain the term solubility. (1 mark)
………………………………………………………………………………………………………
………………………………………………………………………………………………………
b) Calculate the amount of salt crystallized if the solubility of salt X at 40°c is
60g/100g of water. (2 marks)
3 marks