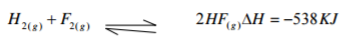KNEC KCSE Chemistry Paper 1 Question Paper / 2016 Pre KCSE
2016 Pre KCSE
Chemistry Paper 1
The table below gives some properties of gases D and E
| GASES | Density | Effect of H2SO4 (aq) | Effect of NaOH(aq) |
| D | Lighter than air | Reacts to form a salt | Dissolves without reacting |
| E | Heavier than air | Not affected | Not affected |
(a) Describe how one would obtain a sample of gas E from a mixture of gases D and E (2mks)
…………………………………………………………………………………………………………
(b) Suggest a possible identity of gas D (1mk)
…………………………………………………………………………………………………………
3 marks
Iron roofing sheets are coated with zinc as sacrificial metal;
(i) What is meant by the term ‘sacrificial’? (1mk)
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
(ii) Give the name given to the process by which iron sheets are coated with zinc. (1mk)
…………………………………………………………………………………………………………
(iii) Zinc is higher than iron in reactivity series yet it does not corrode as fast as iron.
Explain (1mk)
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
3 marks
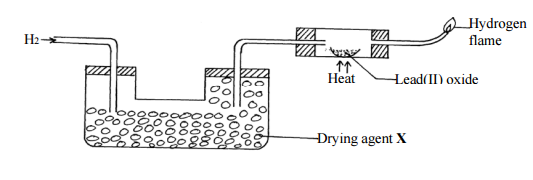
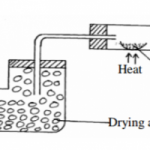
The set-up below was used to investigate the properties of hydrogen gas.
(i) Write an equation for the reaction that takes place in the combustion tube. (1mk)
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
(ii) Suggest a possible drying agent X. (½mk)
…………………………………………………………………………………………………………
(iii) What would happen if the hydrogen gas at the end is not burnt? (1mk)
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
2.5 marks
The table below shows pH values of solutions A to E
| Solution | A | B | C | D | E |
| pH | 3 | 14 | 7 | 6 | 9 |
Which solution;
(a) Contains the largest concentration of hydroxyl ions? (1mk)
…………………………………………………………………………………………………………
(b) Contains the largest concentration of hydrogen ions (1mk)
…………………………………………………………………………………………………………
(c) is likely to be a solution of sodium chloride (1mk)
…………………………………………………………………………………………………………
3 marks
Potassium is isotopic and has a relative atomic mass (R.A.M) of 39.5, work out the percentage
abundance of each isotope. The three isotopes are, 39 K , 40K and 38K (0.01%) A
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
3 marks
An ion of element Q can be represented as
(a) Draw the structure of the ion (2mks)
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
(b) How does its ionic radius compare with its atomic radius (1mk)
3 marks
The electronic configuration for elements represented by letters A, B, C and D are A 2.8.6,
B 2.8.2, C 2.8.1, D 2.8.8
(a) Select the element which forms;
(i) A double charged cation (1mk)
…………………………………………………………………………………………………………
(ii) a soluble carbonate (1mk)
…………………………………………………………………………………………………………
(b) Which element has the shortest atomic radius (1mk)
…………………………………………………………………………………………………………
3 marks
When concentrated hydrochloric acid was electrolysed for a longtime. Two gases were
obtained at the anode ;
(i) Name the two gases (1mk)
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
(ii) Explain why the gases were obtained. (2mks)
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
3 marks
Using dots ( • ) and crosses (X) to represent electrons, draw diagrams to show bonding in;
(a) C2H4 (C=12 H=1) (2mks)
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
(b) Hydro-oxonium ion H3O+ (H=1 O=8) (2mks)
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
4 marks
A student reacted Silver Nitrate and Barium Chloride solutions to prepare two salts.
(i) Write an equation for the reaction that took place (1mk)
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
(ii) Write the ionic equation for the reaction (1mk)
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
(iii) Name the method above use din preparing salts mentioned. (1mk)
…………………………………………………………………………………………………………
4 marks
Give the name and formula of;
(i) A complex cation containing a transition metal (1mk)
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
(ii) A complex anion containing a transition metal (1mk)
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
4 marks
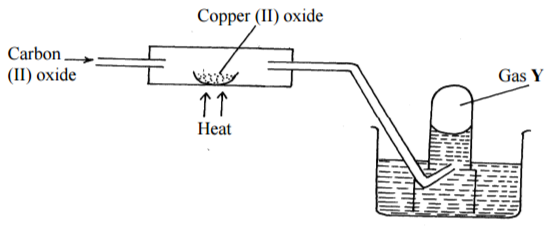
Use the diagram below to answer the questions below:
(i) Identify gas Y (1mk)
…………………………………………………………………………………………………………
(ii) Write an equation for the reaction taking place in the combustion tube. (1mk)
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
(iii) Which is the best place for carrying out the above experiment (1mk)
3 marks
400cm3 of Nitrogen gas diffuses through a porous plug in 70seconds. How long would it
take the same volume of Carbon (IV) oxide to diffuse through the same porous pot?
(C=12, O=16, N=14)
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
3 marks
20.0cm3 of NaOH solution containing 8.0gdm-3 were required for complete neutralization
of 0.118g of a dibasic acid. Calculate the Relative Molecular Mass (R.M.M) of the acid.
(Na=23, O=16, H=1)
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
3 marks
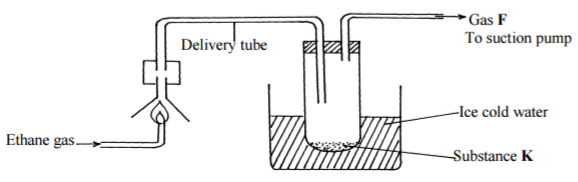
The diagram below shows the combustion of ethane gas. Study it and answer the questions
about it:
(a) Identify substance K (1mk)
…………………………………………………………………………………………………………
(b) Write an equation for the complete combustion of ethane gas. (1mk)
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
(c) The pH of substance K is found to be less than 7. Explain this observation (1mk)
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
3 marks
The structure of hydrogen sulphide can be represented as shown below:
(a) Name the bond type represented by letters X and Y (2mks)
X…………………………………….
Y…………………………….……….
(b) Give a chemical test for hydrogen sulphide gas (1mk)
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
3 marks
In the Haber process, the industrial manufacture of ammonia is given by the following equation ;
(i) Name one source of hydrogen used in the process (1mk)
…………………………………………………………………………………………………………
(ii) Name the catalyst used in the above reaction (1mk)
…………………………………………………………………………………………………………
(ii) What is the effect of increasing temperature on yield of ammonia? Explain (1mk)
3 marks
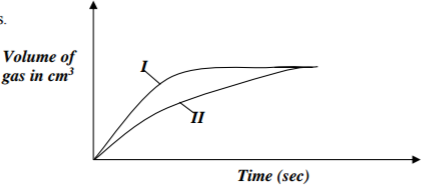
The curves below were obtained when equal volumes of 2M HCl were reacted with 3.0g
of marble chips (CaCO3). In one of the reactions, the acid was warmed before adding the
marble chips.
(a) Write the equation for the reaction (1mk)
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
(b) Identify the curve representing the reaction where the acid was warmed. (1mk)
…………………………………………………………………………………………………………
2 marks
Chlorine gas bubbled into a solution of hydrogen sulphide as shown below:
(i) Explain the observation made in boiling tube. (2mks)
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
(ii) What precautions should be taken in the experiment (1mk)
3 marks
Hydrogen fluorine reacts according to the equation,
(a) On the grid provided below, sketch the energy level diagram for the forward reaction (2mks)
(b) Calculate the molar heat of formation of HF (1mk)
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
(c) What will happen to the yield of HF when the temperature of the above system is
increased? (1mk)
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
3 marks
Study the physical properties of Magnesium and Beryllium. Use it to answer the questions
that follow:
| Element | Be | Mg |
| Mpo C Bp o C Atomic number Atomic radius (nm) |
1280 2450 4 0.086 |
650 1110 12 0.136 |
(a) Explain why Be has a higher m.p than Magnesium (2mks)
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
(b) Write the electron arrangement of Magnesium in the following compound; Mg3(PO4)2 (1mk)
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
3 marks
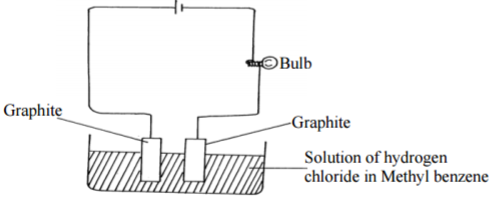
Study the diagram below and answer the questions that follow:
(i)What observation was made during the experiment. Explain? (1½mks)
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
(ii) What observation would be made if the solution of hydrogen in methylbenzene was
replaced with solution of hydrogen chloride in water? Explain (1½mks)
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
3 marks
Explain how you would prepare Aluminium hydroxide, staring with Aluminium sulphate.
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
3 marks
0.28g of iron burns in air to form Iron (II) oxide. Calculate the mass of Iron(II) oxide formed
(O=16, Fe=56)
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
3 marks
The table below gives the solubilities of Potassium Bromine and Potassium Sulphate at 0oC
and at 40oC;
Substance Solubility /100g water
0o
C 40o
C
Potassium Bromide 55 75
Potassium Sulphate 10 12
When aqueous mixture containing 60g of KB and 7g of K2SO4 in 100g water at 80oC was
cooled to 0oC, some crystals were formed;
(i) Identify the crystals (1mk)
…………………………………………………………………………………………………………
(ii) Determine the mass of the crystals formed (1mk)
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
(iii) Name the method used to obtain the crystals (1mk)
…………………………………………………………………………………………………………
(iv) Suggest one industrial application of the method named in (iii) above (1mk)
…………………………………………………………………………………………………………
3 marks
State two environmental problems likely to be found in an area where Sulphur (IV) oxide is
Manufactured.
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
2 marks
Give two advantages of hard water
…………………………………………………………………………………………………………
…………………………………………………………………………………………………………
2 marks