KNEC KCSE Chemistry Paper 3 Question Paper / 2014 Kakamega County Mock
2014 Kakamega County Mock
Chemistry Paper 3
You are provided with
- A mono basic acid A
- 0.2M sodium hydroxide solution B.
- 0.5g of crushed egg shell C
- Methyl orange indicator.
You are required to
- Dilute solution A with distilled water
- Standardize solution A with solution B
- Determine the content of calcium carbonate in the egg shell provided.
Procedure
You are provided with;
- Sodium hydroxide solution labeled solution P
- Carboxylic acid solution labeled solution Q
Procedure
Using a clean burette, place 16cm3 of solution Q into a boiling tube. Take the initial temperature of the solution in the boiling tube and record it in the table shown below. Using a clean measuring cylinder, measure 4 cm3 of solution P into 100 cm3 beaker and add it to solution Q in the boiling tube. Stir the mixture immediately with the thermometer and record in the table II the maximum (final) temperature reached. Repeat the experiment with the other sets of volumes of Q and P in table II and complete it.
(Rinse the thermometer and the boiling tube with distilled water after each experiment)
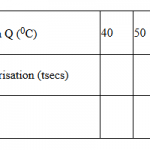
Table II
| Volume of solution Q (cm3) | 16 | 12 | 8 | 6 | 4 | 2 |
| Volume of solution P (cm3) | 4 | 8 | 12 | 14 | 16 | 18 |
| Final temperature (oC) | …. | …. | …. | …. | …. | …. |
| Initial temperature(oC) | …. | …. | …. | …. | …. | …. |
| Change in temperature(∆T) | …. | …. | …. | …. | …. | …. |
(6 MARKS)
a) On the grid provided, plot a graph of ∆T (vertical axis) against the volume of sodium hydroxide, solution A. (3 MARKS)
b) From the graph, determine the volume of sodium hydroxide, solution A required to neutralize the carboxylic acid. (1 MARK) ……………………………………………………………………………………………………………………………………………………………………………
c) Calculate the volume of carboxylic acid, solution used for neutralization. (6 MARKS)
……………………………………………………………………………………………………………………………………………………………………………
d) Calculate the
i. Ratio between the volumes of solution A and C (6 MARKS) ………………………………………………………………………………………………………………………………………………………………
………………………………………………………………………………………………………………………………………………………………
ii. Concentration in moles per litre of the carboxylic acid, solution C.
(Assume that the volume ratio is the same as the mole ratio) (6 MARKS) ………………………………………………………………………………………………………………………………………………………………
………………………………………………………………………………………………………………………………………………………………
………………………………………………………………………………………………………………………………………………………………
28 marks