KNEC KCSE Chemistry Paper 3 Question Paper / 2016 KCSE MOKASA Joint Examination
2016 KCSE MOKASA Joint Examination
Chemistry Paper 3
You are provided with;
• Solid Q, 2.0g of impure sodium carbonate (contaminated with sodium chloride).
• Solution R, hydrochloric acid solution, containing 2.07g of the acid in 500 cm3 of solution.
You are required to determine the percentage impurity in solid Q.
Procedure:
(i) Place all solid Q in a beaker and add 100cm3 of distilled water. Stir well a glass rod.
(ii) Transfer the solution into a 250 cm3 volumetric flask and top it up to the mark with
distilled water. Shake well and label as solution Q.
(iii) Fill a burette with solution R.
(iv) Pipette 25.0 cm3 of solution Q into a conical flask. Add three drops of methyl orange
indicator.
(v) Titrate solution Q against solution R from the burette. Record the results in the table
below.
(vi) Repeat the titration two more times and complete the table.
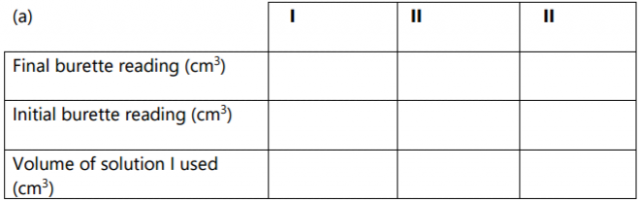
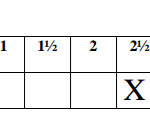
Table 1
(a) Determine the average volume of solution R used. (1 mark)
(b) Calculate the concentration of solution R in moles per litre. (2 marks)
(H = 1.0, Cl = 35.5)
(c) Calculate the number of moles of the acid in solution R that reacted. (1 mark)
(d) Write an equation for the reaction that occurs. (1 mark)
(e) Calculate the number of moles of sodium carbonate in 25 cm3 of solution Q that
reacted. (1 mark)
(f) Calculate the mass of sodium carbonate in 250 cm3 of solution Q. (2 marks)
(C = 12.0, O = 16, Na = 23)
(g) Find the percentage of mass of the impurity, sodium chloride, in solid Q. (2 marks)
15 marks
Your are required to investigate the effect of change in concentration on the reaction rate
between sodium thiosulphate solution C and dilute hydrochloric acid solution D. When
hydrochloric acid is added to sodium thiosulphate sulphur is deposited.
𝑁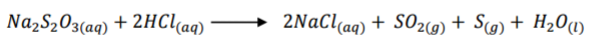
reaction. Solution C contains 0.08 moles of sodium thiosulphate in one litre of solution.
Procedure:
(i) Measure 40 cm3 of solution C and pour it into a 100 cm3 glass beaker.
(ii) Mark a cross (X) on a white paper. Place the beaker containing solution C over the
cross on the paper.
(iii) Measure 10 cm3 of solution D add it to the solution C in the beaker. Start the
stopwatch immediately. Observe the cross on the white paper from the top of the
beaker and record the time taken for it to be obscured (to disappear from view).
(iv) Repeat the experiment using different volumes of solution C as indicated in the
following table and in each case water is added to make a total of volume of 40 cm3
The same volume of hydrochloric acid is added in each case.
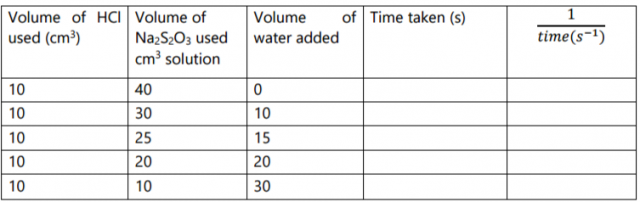
Complete the table below. (5 marks)
On the grid provided plot a graph of the reciprocal of time 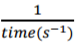
of solution C used. (3 marks)
III. Explain the shape of the graph in terms of rates of reaction. (1 mark)
10 marks
I.
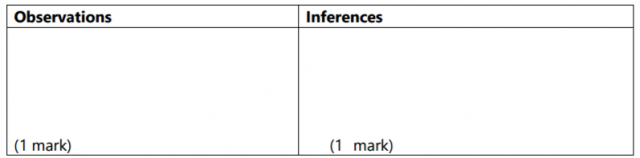
(a) You are provided with solid K. Carry out the tests below. Write your observations
and inferences in the spaces provided.
Place all of solid K in a boiling tube, add about 10cm3 of distilled water and shake
until all the solid dissolves. Divide the solution into 4 portions.
(i) To the first portion in a test-tube, add a few drops of sodium hydroxide until
in excess. Retain the mixture for procedure (b).
(i) Warm the mixture in (a) above and test any gases produced using red and blue litmus papers.
(iii ) To the third portion, add about equal volume of freshly lead (II) nitrate
solution followed by a few drops of dilute nitric (V) acid.
(iv) To the fourth portion add Barium nitrate solution.
II. You are provided with substance Z. Carry out the tests below. Write your
observations and inferences in the spaces provided.
(a) Scoop a little of solid Z using a clean spatula and burn it in a Bunsen burner
flame.
Divide the remaining amount into two portions.
(b) To the first portion, add water and shake.
(c) To the second portion, add potassium manganate (VII) and warm.
(d) To a little amount of Z, add sodium carbonate.
15 marks