KNEC KCSE Chemistry Paper 3 Question Paper / 2016 Pre KCSE
2016 Pre KCSE
Chemistry Paper 3
You are provided with:
Solution M 0.2M hydrochloric acid,
Solution F containing 15.3g per litre of basic compound G2X.H2O.
You are required to determine the relative atomic mass of G.
PRECEDURE:
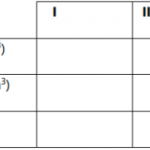
Place solution M in a burette ,pipette 25cm3 of solution F into a 250cm3 conical flask. Add
two drops of methyl orange indicator and titrate. Record your results in the table below.
Repeat the procedure two more times and complete table I.
(4mks)
ii) What is the average volume of solution M.? (1mk)
…………………………………………………………………………………………………
…………………………………………………………………………………………………
b) Given that one mole of F reacts with 2moles of M. Calculate the;
i) number of moles the basic compound,G2X, 10H2O in the volume of solution
F used. (2mks)
…………………………………………………………………………………………………
…………………………………………………………………………………………………
…………………………………………………………………………………………………
…………………………………………………………………………………………………
ii) Concentration of solution F in mole per litre. (2mks)
…………………………………………………………………………………………………
…………………………………………………………………………………………………
…………………………………………………………………………………………………
…………………………………………………………………………………………………
iii) Relative formula mass of the basic compound, G2X.10H2O. (1 ½ mks)
…………………………………………………………………………………………………
…………………………………………………………………………………………………
…………………………………………………………………………………………………
iv) relative atomic mass of G (Relative formula Mass of X=60 , atomic mass of H=1.0 ,
O=16.0). (1 ½ mks)
…………………………………………………………………………………………………
…………………………………………………………………………………………………
…………………………………………………………………………………………………
12 marks
You are provided with:
- 1.899g of solid P, solid P is adiabatic acid H2X.
- 0.5M Solution of the dibasic acid , H2X , Solution V.
- Sodium hydroxide, Solution K.
You are required to determine:
a) i) the molar heat of solid P.
ii) the heat of reaction of one mole of the dibasic acid with sodium
hydroxide.
b) Calculate the heat of reaction of solid H2X with aqueous sodium hydroxide.
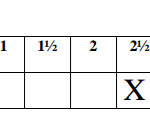
PROCEDURE I
Place 30cm3 of distilled water into a 100ml beaker. Measure the initial temperature of the
water and record it in the table II below. Add all the solid P at once; stir the mixture carefully
with the thermometer until all the solid dissolves. Measure the final temperature reached and
records it in the table II
a) Determine the change in temperature ∆T1 (1½mks)
…………………………………………………………………………………………………
b) Calculate the:
i) heat change when H2X dissolves in water, (Assuming the heat capacity
of the solution is 4.2J-1g-1 K -1 and density is 1g/cm3) (2mks)
ii) number of moles of the acid that were used. (Relative formula mass of
H2X is 126) (1mk)
…………………………………………………………………………………………………
…………………………………………………………………………………………………
…………………………………………………………………………………………………..
iii) molar heat of solution ∆H1 solution of the acid H2X. (1mk)
…………………………………………………………………………………………………..
…………………………………………………………………………………………………..
…………………………………………………………………………………………………..
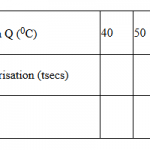
PROCEDURE II.
Place 30cm3 of solution V into a 100cm3 beaker. Measure the initial temperature and record it
in table III below. Measure 30cm3 of sodium hydroxide, solution K.Add all of the 30cm3 of t
of solution K at once to V in the beaker. Stir the mixture with the thermometer. Measure the
final temperature reached and record it in table III.
b) Determine the change in temperature, ∆T2. ( ½ mk)
…………………………………………………………………………………
c) Determine the:
i) heat change for the reaction (Assume the heat capacity of the solution is
4.2J-1g-1K -1 and density is 1g/cm3 (2mks)
……………………………………………………………………………
………………………………………………………………………….
ii) Number of moles of the acid used (H2X). (1mk)
……………………………………………………………………..
…………………………………………………………………….
………………………………………………………………………
iii) Heat of reaction, H 2 of one mole of the acid H2X with sodium hydroxide
(1mk)
…………………………………………………………………………………………
………………………………………………………………………………………..
…………………………………………………………………………………………
………………………………………………………………………………………..
d) Given that, ∆H1 is the heat for reaction
∆ H2 is the heat for the reaction
Calculate ∆H3 for the reaction 
……………………………………………………………………………
……………………………………………………………………………
……………………………………………………………………………
……………………………………………………………………………
…………………………………………………………………………
14 marks
You are provided with solid S. Carry out the tests below and record your observations
and inferences in the spaces provided.
a) Place about one third of solid S in a dry test tube. Heat the solid gently and the
strongly. Test any gases produced with blue and red litmus papers.
b) Dissolve the remaining portion of solid S in 8cm3 of distilled water.
i) Divide the solution into the first portions, to the first portion, add
aqueous sodium hydroxide drop wise until in excess.
ii) To the second portion , add aqueous ammonia dropwise in excess.

v) To the fifth portion, add about 2ml of hydrogen peroxide then about
1cm3 of sodium hydroxide solution.
14 marks