KNEC KCSE Past Papers 2019 Chemistry Paper 1 (233/1)
Kenya certificate of Secondary Education
2019 Chemistry paper 1
1. An atom of element A has mass number 39 and 19 protons.
(a) Write the electron arrangements of the atom (1 mark)
(b) State the period and group to which element A belongs Group ………………………………….(½ mark)
Period …………………………………..(½ mark)
(c) State whether the element is a metal or a non-metal. (1 mark)
2. Describe how an increase in concentration increases the rate of a reaction. (2 mark)
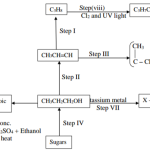
3. The flow chart in Figure 1 represents some stages in the extraction of copper metal. Study it and answer the questions that follow.
(a) Identify :
(i) The copper ore (1 mark)
(ii) Process B (½ mark)
(iii) Solid C (½ mark)
(b) Write an equation for the reaction that forms the slag. (1 mark)
4. A monomer has the following structure.
CH= CH2 ∣ C6H5
(a) Draw the structure of its polymer that contains three monomers.(1 mark)
(b) A sample of the polymer formed from the monomer has a molecular mass of 4992.
Determine the number of monomers that formed the polymer (C= 12; H= 1.0). (2 marks)
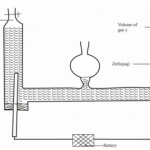
5. Hydrogen has can be prepared by passing steam over heated magnesium ribbon as shown in the figure 2.
(a) Write an equation for the reaction that produces hydrogen gas. (I mark)
(b) Explain why the delivery tube must be removed from beneath the water before heating is stopped. (1 mark)
(c) Explain why sodium metal is not suitable for this experiment. (I mark)
6. A farmer intended to plant cabbages in his farm. He first tested the pH of the soil and found it to be 3.0.
If cabbages do well in alkaline soils, explain the advice that would be given to the farmer in order to realise a high yield.(2 marks)
7. A solution contains 40.3g of substance XOH per litre .250.0cm3of this solution required 30.0cm3 of 0.3M sulphuric(VI)acid for complete neutralisation. (a) Calculate the number of moles of XOH that reacted. (½ mark)
(b) Determine the relative atomic mass of X. (1½ mark)
8. Table 1 shows the properties of two chlorides, D and E.
Table 1
| Chloride | Melting Points(°C) | Electrical Conductivity (liquid) |
|---|---|---|
| D | 1074 | Good |
| E | 203 | Poor |
(a) State the type of bond present in:
(i) D…………………..(1 mark)
(ii) E…………………..(1 mark)
(b) Explain in terms of structure and bonding, the difference in electrical activity of the chlorides D and E. (1 mark)
9. Sulphur(IV) oxide is prepared in the laboratory using the set-up in Figure 3.
Study it and answer the questions that follow.
(a) Identify substance F. (1 mark)
(b) Write an equation for the reaction that takes place in the flask. (1 mark)
(c) State the purpose of liquid G. (1 mark)
The graph in Figure 4 was obtained when a certain substance was heated and its temperature recorded at regular intervals.
(a) State the purity of the substance. (1 mark)
(b) Explain the answer in (a). (1 mark)
11. Ethene is prepared in the laboratory by dehydration of ethanol.
(a) Name a suitable dehydrating agent used in this process. (1 mark)
(b) State the condition necessary for the reaction to occur. (1 mark)
(C) Write an equation for the dehydration process. (1 mark)
112.A boiling yube filled with chlorine was inverted in a trough containing the same ssolution and the set-up left in sunlight for about 2 hours.
(a) State the observation made in the boiling tube ( 1 mark)
(b) Explain the observation made in (a) (1 mark)
(c) Write an equation for the reaction that occurred in the boiling tube (1 mark)
13. 5 g of calcium carbonate was strongly heated to a constant mass.
Calculate the mass of the solid residue formed (Ca = 40.0; C = 12.0; 0 = 16.0). (2 marks)
14. During laboratory preparation of oxygen, manganese(IV) oxide is added to reagent 11.
(a) Name reagent H. (1 mark)
(b) State the role of manganese(IV) oxide in this experiment. (1 mark)
(c) Write the equation for the reaction that takes place. ( I mark)
15. Figure 5 shows an apparatus used to separate a mixture of water and hexene.
(a) Name the apparatus in Figure 5. (1 mark)
(b) State the principle by which the mixture of the two liquids is separated (1 mark)
(c) Identify the liquids, R and S if the density of hexene is 0.66 g/cm3.
(i) R …………… (½ mark)
(ii) S …………………. (½ mark)
16.(a) Complete the following table.(2 mark)
| Solution | pH | Nature of solution |
|---|---|---|
| H | 1.0 | |
| I | Neutral | |
| J | Weak acid | |
| K | 13.0 |
(b) Explain why a solution of ammonia in methylbenzene has no effects on red litmus paper while in aqueous ammonia red litmus paper turns blue. (1 mark)
17. The heat of solution and hydration energy of potassium chloride is — 17.2 kJ and —689 kJ respectively.
Calculate the lattice energy of potassium chloride. (2 marks)
18. Use the information in Table 2 to answer the questions that follow.
| Bond | Bond energy(KJ mol(<sub>-1</sub> |
|---|---|
| C-H | 412 |
| CI-CI | 242 |
| C-CI | 338 |
| H-C1 | 431 |
19. Given that the Eθ of CU(s),CU2+(aq)is + 0.34V and that 0f Zn(s)/Zn2+(aq)is- 0.76V,draw a labelled diagram of zinc and copper electrochemical cell. (3 marks)
20. During labomtory preparation of carbon(IV) oxide g tS. substance L in a conical flask.
(a) Identify substance L. (1 mark)
(b) Write an equation that produces carbon(IV) oxide. (1 mark)
(c) State the observations made when the gas produced WHS bubbled through calcium hydroxide solution for a long time. (1 mark)
21. Study the information in Table 3 and use it to answer the questions that follow. (I mark)
| Elements | Na | Mg | Al | Si | P | S | Cl |
|---|---|---|---|---|---|---|---|
| Atomic Numbers | 11 | 12 | 13 | 14 | 15 | 16 | 17 |
| Atomic radii(nm) | 0.157 | 0.136 | 0.125 | 0.117 | 0.110 | 0.104 | 0.099 |
(a) Explain the trend in atomic radii from sodium to chlorine. (1 mark)
(b) Explain how the chloride of aluminium differs from those of other metals in the period.(2 marks)
2. The diagram in figure 6 shows radiations emitted by a radioactive sample.
(a) Identify radiations:
(i) M ……………. (1 marks)
(ii) N ……………… (1 marks)
(b) Explain what would happen when a sheet of paper is placed in the path of the two radiations. (1 mark)
16/8 X 18/8 X are isotopes of element X.
They occur naturally in the ratio of 9:1 respectively.
Calculate the relative atomic mass of element X. (2 marks)
24. Starting with copper turnings, describe how a sample of copper (II) Sulphate crystals can be prepared in the laboratory. (2 marks)
25. Chemical tests were carried out on separate samples of water drawn from the same source. The observations made were recorded as shown in Table 4.
| Test | Observation |
|---|---|
| (i) Addition of aqueous calcium chloride | No white precipitate |
| (ii) Addition of dilute sulphuric(VI) acid | No effervescence, colourless solution |
| (iii) Addition of a few drops of acidified barium nitrate |
No white precipitate |
| (iv) Addition of aqueous ammonia | White precipitate dissolves |
State the inferences made in reactions:
(i)……………………… (1 mark)
(ii)……………………….. (1 mark)
(iii)…………………………(1 mark)
26. 140cm3of nitrogen gas diffuses through a membrane in 70 seconds. How long will it take 200 cm3of carbon(iv) oxide gas to diffuse through the same membrane under the same conditions of temperature and pressure (3 marks)
27. When burning magnesium ribbon is introduced into a gas jar full of nitrogen, it continues to burn producing a greenish yellow powder.
(a) Write an equation for the reaction between nitrogen and magnesium. (I mark)
(b) Explain why magnesium continues to bum in nitrogen but sulphur does not. (2 marks)
(c) State one use of nitrogen. (I mark)
28. Draw in the space provided a labelled diagram of the set-up of the apparatus that can be used to electrolyse molten lead(II) bromide. (3 marks)
29. Name an appropriate apparatus that is used to prepare standard solutions in the laboratory.( I mark)
Questions and Answers
Kenya certificate of Secondary Education
2019 Chemistry paper 1
1. An atom of element A has mass number 39 and 19 protons.
(a) Write the electron arrangements of the atom (1 mark)
- 2.8.8.1(b) State the period and group to which element A belongs Group 1(½ mark)
Period 3(½ mark)
(c) State whether the element is a metal or a non-metal. (1 mark)
- Metal2. Describe how an increase in concentration increases the rate of a reaction. (2 mark)
As the concentration increases, the number of reacting particles increases leading to increase in effective collisions. This leads to increase in the rate of reaction.
3. The flow chart in Figure 1 represents some stages in the extraction of copper metal. Study it and answer the questions that follow.
(a) Identify :(i) The copper ore (1 mark)
- Copper pyrites/CuFeS2(ii) Process B (½ mark)
- Froth floatation(iii) Solid C (½ mark)
- Copper(1) suphide/Cu2S(b) Write an equation for the reaction that forms the slag. (1 mark)
FeO(s) + SiO2 (s) —+ FeSiO3(I)
4. A monomer has the following structure.
CH= CH2 ∣ C6H5
(a) Draw the structure of its polymer that contains three monomers.(1 mark)
(b) A sample of the polymer formed from the monomer has a molecular mass of 4992.Determine the number of monomers that formed the polymer (C= 12; H= 1.0). (2 marks)
RFM of monomer = (12 x 8) + 8 =104
Numbers of monomers = 4992/104 = 48units
5. Hydrogen has can be prepared by passing steam over heated magnesium ribbon as shown in the figure 2.
(a) Write an equation for the reaction that produces hydrogen gas. (I mark)(b) Explain why the delivery tube must be removed from beneath the water before heating is stopped. (1 mark)
- To prevent suck-back of water which would crack the boiling tube. Sodium is a very reactive metal hence reacts explosively with steam.(c) Explain why sodium metal is not suitable for this experiment. (I mark)
- To prevent suck-back of water which would crack the boiling tube.
- Sodium is a very reactive metal hence reacts explosively with steam.6. A farmer intended to plant cabbages in his farm. He first tested the pH of the soil and found it to be 3.0.
If cabbages do well in alkaline soils, explain the advice that would be given to the farmer in order to realise a high yield.(2 marks)
- Add calcium oxide /1ime to raise the soil pH. Calcium oxide is a basic oxide hence dissolves in water in the soil to form alkaline solution that reacts with acidic soil raising soil pH.7. A solution contains 40.3g of substance XOH per litre .250.0cm3of this solution required 30.0cm3 of 0.3M sulphuric(VI)acid for complete neutralisation.
(a) Calculate the number of moles of XOH that reacted. (½ mark)
H2SO4(g) + 2XOH(aq) —› X SO4(aq) + 2H2 O(l)
Moles of H2SO4 = 30 x 0.3/1000 = 0.009 moles
Moles of XOH 2 x 0.009 0.018 moles
(b) Determine the relative atomic mass of X. (1½ mark)
Molarity of XOH 0.018 x1000/25
= 0.72M
R.F.M = g /1/molarity = 40.3/0.72 = 55.972 =56
0.72 x +16 +1 = 56
x = 56 – 17
x = 39
8. Table 1 shows the properties of two chlorides, D and E.
Table 1
Chloride Melting Points(°C) Electrical Conductivity
(liquid)D 1074 Good E 203 Poor (a) State the type of bond present in:
- D Ionic/electrovalent.
- Covalent (Van der Waals)(b) Explain in terms of structure and bonding, the difference in electrical activity of the chlorides D and E. (1 mark)
- Chloride of D giant ionic; when in molten state the ions are mobile hence conducts electric current. E is giant molecular and therefore does not have mobile ions to carry electric current.9. Sulphur(IV) oxide is prepared in the laboratory using the set-up in Figure 3.
Study it and answer the questions that follow.
(a) Identify substance F. (1 mark)
- Dilute hydrochloric acid / dilute HC1 acid
- Dilute sulphuric (VI) acid/dilute H2SO4(b) Write an equation for the reaction that takes place in the flask. (1 mark)
Na2SO3(s) + 2HC1(Sq) SO z(8) + 2NaCl(dQ) + H2O (1) or Na2SO3(s) +H2SO4 (aq) —+ Na SO4 (aq) + H O(l) +SO2(g)
(c) State the purpose of liquid G. (1 mark)
- To dry the gasThe graph in Figure 4 was obtained when a certain substance was heated and its temperature recorded at regular intervals.
(a) State the purity of the substance. (1 mark)
- Impure(b) Explain the answer in (a). (1 mark)
- The substance does not have a sharp melting point or boiling point11. Ethene is prepared in the laboratory by dehydration of ethanol.
(a) Name a suitable dehydrating agent used in this process. (1 mark)
- Concentrated sulphuric(VI) acid Al203 H3PO4(b) State the condition necessary for the reaction to occur. (1 mark)
- Temperature 160°C —l80°C(C) Write an equation for the dehydration process. (1 mark)
CH3CH2OH H2SO4 CH2CH2 + H2O
12. A boiling yube filled with chlorine was inverted in a trough containing the same solution and the set-up left in sunlight for about 2 hours.
(a) State the observation made in the boiling tube ( 1 mark)
- The colour of the solution changes from yellow to colourless.
- Colourless gas collected/ level of solution drops.(b) Explain the observation made in (a) (1 mark)
The sunlight decomposed chloric(I) / hypochlorous acid to oxygen and hydrochloric acid.
(c) Write an equation for the reaction that occurred in the boiling tube (1 mark)
2HOCl(aq) —+ 2HCl(aq) + 02 (g)
13. 5 g of calcium carbonate was strongly heated to a constant mass.
Calculate the mass of the solid residue formed (Ca = 40.0; C = 12.0; 0 = 16.0). (2 marks)
CaCO,(s) —› CaO(s) + CO2(g)
No. of moles of CaCO3 = 5/100 = 0.05 moles
100 Moles CaO = 0.05
Mass of CaO = 0.05 x 56
= 2.8g
14. During laboratory preparation of oxygen, manganese(IV) oxide is added to reagent 11.
(a) Name reagent H. (1 mark)
- Hydrogen peroxide(b) State the role of manganese(IV) oxide in this experiment. (1 mark)
- Catalyst, to speed up the production of oxygen gas.(c) Write the equation for the reaction that takes place. ( I mark)
2H2O2 (1) —+ 2H O(1) + 02 (g)
15. Figure 5 shows an apparatus used to seperate a mixture of water and hexene.
(a) Name the apparatus in Figure 5. (1 mark)
- Separating funnel(b) State the principle by which the mixture of the two liquids is separated (1 mark)
- Immiscibility/different densities(c) Identify the liquids, R and S if the density of hexene is 0.66 g/cm3.
(i) R Hexene (½ mark)
(ii) S Water (½ mark)
16.(a) Complete the following table.(2 mark)
Solution pH Nature of solution H 1.0 I Neutral J Weak acid K 13.0 (b) Explain why a solution of ammonia in methylbenzene has no effects on red litmus paper while in aqueous ammonia red litmus paper turns blue. (1 mark)
- Ammonia in methylbenzene is molecular/ does not dissociate, while it ionizes in water to form ions.17. The heat of solution and hydration energy of potassium chloride is — 17.2 kJ and —689 kJ respectively.
Calculate the lattice energy of potassium chloride. (2 marks)
Hsoln =H latt + Hhyd
-17.2 = AHlatt + (- 689) AHlatt = +689 -17.2
= +671.8kJmol
18. Use the information in Table 2 to answer the questions that follow.
Bond Bond energy(KJ mol(<sub>-1</sub> C-H 412 CI-CI 242 C-CI 338 H-C1 431 19. Given that the Eθ of CU(s),CU2+(aq)is + 0.34V and that 0f Zn(s)/Zn2+(aq)is- 0.76V,draw a labelled diagram of zinc and copper electrochemical cell. (3 marks)
20. During laboratory preparation of carbon(IV) oxide g tS. substance L in a conical flask.(a) Identify substance L. (1 mark)
Calcium carbonate/CaCO3/Marble chips / ( any other suitable carbonate)
(b) Write an equation that produces carbon(IV) oxide. (1 mark)
CaCO3(S) + 2HCl(aq) —› CaCl2 (aq) + H2O(1) + CO2 (g)
(c) State the observations made when the gas produced WHS bubbled through calcium hydroxide solution for a long time. (1 mark)
- White precipitate formed which dissolves in excess to form a colourless solution.21. Study the information in Table 3 and use it to answer the questions that follow. (I mark)
Elements Na Mg Al Si P S Cl Atomic Numbers 11 12 13 14 15 16 17 Atomic radii(nm) 0.157 0.136 0.125 0.117 0.110 0.104 0.099 (a) Explain the trend in atomic radii from sodium to chlorine. (1 mark)Atomic radii decreases across the period .Across the period the number of protons increases increasing the nuclear attraction for the outermost electrons contributing to decrease in atomic radii.
(b) Explain how the chloride of aluminium differs from those of other metals in the period.(2 marks)
AlCl3 is molecular/ covalent. It differs from other metal chlorides because it exits as a dimmer. Two molecules of AlCl3 pair through co- ordinate bonds while the other metal chlorides are ionic.
OR AICl3 hydrolyzes in water while the other chlorides do not.
22. The diagram in figure 6 shows radiations emitted by a radioactive sample.
(a) Identify radiations:(i) M Alpha α(1 marks)
(ii) N Beta β(1 marks)
(b) Explain what would happen when a sheet of paper is placed in the path of the two radiations. (1 mark)
The alpha (α) particles will be stopped while beta (β) particles will penetrate the sheet of paper. This is because beta particles have higher penetrating power than alpha particles.
23.16/8 X 18/8 X are isotopes of element X.
They occur naturally in the ratio of 9:1 respectively.
Calculate the relative atomic mass of element X. (2 marks)
R.A.M — (9/10×16) +(1/10×18)
— 14.4 +1.8
= 16.2
24. Starting with copper turnings, describe how a sample of copper (II) Sulphate crystals can be prepared in the laboratory. (2 marks)
- Heat copper turnings in air to form copper(II) oxide.
- Add dilute sulphuric(VI) acid filter to obtain copper(II) sulphate solution. Heat to
- saturation and allow to cool for crystals to form. Dry between filter papers.25. Chemical tests were carried out on separate samples of water drawn from the same source. The observations made were recorded as shown in Table 4.
Test Observation (i) Addition of aqueous calcium chloride No white precipitate (ii) Addition of dilute sulphuric(VI) acid No effervescence, colourless solution (iii) Addition of a few drops of acidified
barium nitrateNo white precipitate (iv) Addition of aqueous ammonia White precipitate dissolves State the inferences made in reactions:
- Pb2+, Ag+, CO2-3 and SO2-4 absent
- CO2-4 absent
- SO2-4 absent26. 140cm3of nitrogen gas diffuses through a membrane in 70 seconds. How long will it take 200 cm3of carbon(iv) oxide gas to diffuse through the same membrane under the same conditions of temperature and pressure (3 marks)
27. When burning magnesium ribbon is introduced into a gas jar full of nitrogen, it continues to burn producing a greenish yellow powder.(a) Write an equation for the reaction between nitrogen and magnesium. (I mark)
- 3Mg(s) + N2 (g) —› Mg3N2 (s)(b) Explain why magnesium continues to bum in nitrogen but sulphur does not. (2 marks)
Burning magnesium produces a lot of heat that is enough to break N-N triple bond hence reacts with it while burning of sulphur produce little heat not enough to break N – N triple bond.
(c) State one use of nitrogen. (I mark)
- In refrigeration e.g.storage of semen for artificial insemination
- Manufacture of ammonia; Haber process
- In light bulbs28. Draw in the space provided a labelled diagram of the set-up of the apparatus that can be used to electrolyse molten lead(II) bromide. (3 marks)
29. Name an appropriate apparatus that is used to prepare standard solutions in the laboratory.( I mark)Volumetric flask