KNEC KCSE Past Papers Chemistry 2015
CHEMISTRY
Paper 1
(THEORY)
1 (a) Give the name of the first member of the alkene homologous series. (1 mark)
(b) Describe a chemical test that can be used to distinguish butanol from butanoic acid. (2 marks)
2 (a) Name the raw material from which sodium is extracted. (l mark)
(b) Give a reason why sodium is extracted using electrolysis. (1 mark)
(c) Give two uses of sodium metal. (l mark)
3 (a) What is meant by lattice energy? (1 mark)
(b) Study the energy level diagram below and answer the question that follows:
What type of reaction is represented by the diagram? (1 mark)
4 (a) State the Boyles law. (1 mark)
(b)A gas occupies 500 cm3 at 27°C and 100,000 Pa. What will be its volume at 0°C and 101325 Pa? (2 marks)
5 Calculate the mass of Zinc oxide that will‘just neutralise dilute nitric (V) acid containing 12.6 g of nitric (V) acid in water. (Zn = 65.0; O =l6.0, H = 1.0, N = 14.0). (3 marks)
6 Describe how sodium carbonate is used to remove Water hardness. (2 marks)
7 Hydrogen chloride gas can be prepared by reacting sodium chloride with an acid.
(a) Write an equation for the reaction between sodium chloride and the acid. (1 mark)
(b) Give two chemical properties of hydrogen chloride gas. (1 mark)
(c) State two uses of hydrogen chloride. (1 mark)
8 When solid A was heated strongly, it gave off water and a solid residue. When water was added to the solid residue, the original solid A, was formed.
(a) What name is given to the process described? (1 mark)
(b) Give one example of solid A. (1 mark)
9 The set up below was used to investigate the reaction between dry hydrogen gas and copper (II) oxide.
(a) Name substance A. (l mark)
(b) State the observation made in the combustion tube. (1 mark)
(c) Explain the observation stated in (b) above. (1 mark)
10 The atomic number of an element, T is 15.
(a) Write the electronic configuration of the ion T3-. (1 mark)
(b) Write the formula of an oxide of T. (1 mark)
11 Dilute sulphuric (VI) acid was electrolysed using platinum electrodes. Name the product formed at the anode and give a reason for your answer. (2 marks)
12 The curve shown below shows the variation of time against temperature for the reaction between sodium thiosulphate and hydrochloric acid.
(a) Write the equation for the reaction between sodium thiosulphate and dilute hydrochloric acid. (1 mark)
(b) Explain the shape of the curve. (2 marks)
13 Dry ammonia and dry oxygen were reacted as shown in the diagram below.
(a) What is the purpose of the glass wool? (1 mark)
(b) What products would be formed if red hot platinum was introduced into a mixture of ammonia and oxygen? (1 mark)
14 The table below shows behaviour of metals R, X, Y and Z. Study it and answer the questions that follow:
(a) Arrange the metals in the order of reactivity starting with the most reactive. (2 marks)
(b) Name a metal which is likely to be: (1 mark)
15 Given the following substances: wood ash, lemon juice and sodium chloride.
(a) Name one commercial indicator that can be used to show whether wood ash, lemon juice and sodium chloride are acidic, basic or neutral. (1 mark)
(b) Classify the substances in 15(a) above as acids, bases or neutral. (2 marks)
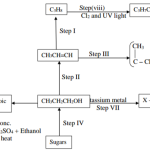
16 The flow chart below shows various reactions of aluminium metal. Study it and answer the questions that follow:
(a) (i) Other than water, name another reagent that could be R. (1 mark)
(ii) Write the formula of reagent Q. (1 mark)
(b) Write an equation for the reaction in step 5. (1 mark)
17 (a) One of the allotropes of sulphur is rhornhic sulphur, name the other allotrope. (1 mark)
(b) Concentrated sulphuric (VI) acid reacts with ethanol and copper. State the property of the acid shown in each case. (2 marks)
18 Study the standard electrode potentials in the table below and answer the questions that follow.
(a) Which of the metals is the strongest reducing agent? (1 mark)
(b) What observations will be made if a silver coin was dropped into an aqueous solution of copper (II) sulphate? Explain. (2 marks)
19 A radioactive substance weighing M kg took 1900 years for the original mass to reduce to l5 kg. Given that the half life of the radioactive substance is 380 years;
(a) Determine the original mass of the radioactive substance. (2 marks)
(b) State two uses of radioactivity in medicine. (1 mark)
20 A crystal of iodine, heated gently in a test tube gave off a purple vapour.
(a) Write the formula of the substance responsible for the purple vapour. (1 mark)
(b) What type of bond is broken when the iodine crystal is heated gently? (1 mark)
(e) State one use of iodine. (1 mark)
21 Describe how samples of lead (II) sulphate, ammonium chloride and sodium chloride can be obtained from a mixture of the three. (3 marks)
22 Study the flow chart below and use it to answer the questions that follow.
(a) Name process T
(b) Give the formula of W.
(c) State two uses of X.
23 The table below is part of the periodic table. The letters are not the actual symbols of the elements. Study it and answer the questions that follow.
(a) Select an element which is stored in paraffin in the laboratory. (l mark)
(b) How do the ionic radii of E and I compare? Explain. (2 marks)
24 The graph below is a cooling curve for water. Study it and answer the questions that follow.
(a) Explain what happens to the molecules of water in the region BC in terms of kinetic theory. (2 marks)
(b) In what state is the water in the region DE? (1 mark)
25 Starting with barium nitrate solution, describe how a pure sample of barium carbonate can be prepared in the laboratory. (3 marks)
26 A hydrocarbon contains 14.5% of hydrogen. If the molar mass of the hydrocarbon is 56, determine the molecular formula of the hydrocarbon. (C = 12.0; H = 1.0) < (3 marks)
27 (a)Describe how carbon (IV) oxide can be distinguished from Carbon II Oxide using calcium hydroxide solution. (2 marks)
(b)What is the role of carbon (IV) oxide in fire extinguishing? (l mark)
28 (a)State one source of alkanes. (1 mark) (b)Ethane gas was reacted with 1 mole of bromine gas. State one observation made during this reaction. (1 mark)
29 An electric current was passed through several substances and the results obtained recorded in the table below.
Which of these substances is likely to be: (a) magnesium (1 mark)
(b) hexane (1 mark)
(c) lead (II) bromide (1 mark)
CHEMISTRY
Paper 2
(THEORY)
1 (a) (i) Carbon (IV) oxide is present in soft drinks. State two roles of carbon (IV) oxide in soft drinks. (l mark)
(ii) Explain the observation made when a bottle containing a soft drink is opened. (2 marks) ;
(iii) Carbon (IV) oxide dissolves slightly in water to give an acidic solution. Give the formula of the acid. (1 mark)
(b) Zinc oxide can be obtained by heating zinc nitrate. A student healed 5.76 g of zinc nitrate.
(i) Write an equation for the reaction that occurred. (1 mark)
(ii) Calculate the total volume of gases produced. (Molar gas volume is 24 dm3; Zn = 65.4; O = 16.0; N = 14.0). (4 marks)
(iii) Identify the element that is reduced when zinc nitrate is heated. Give a reason. (2 marks)
2 (a) Draw the structure of the following compounds. (2 marks)
(i) Butanoic acid;
(ii) Pent-2-cue.
(b) Explain why propan-l-ol is soluble in water While prop-1-ene is not. (Relative molecular mass of propan-1-ol is 60 while that of prop-1-ene is 42). (2 marks)
(c) What would be observed if a few drops of acidified potassium manganate (VII) were added to oil obtained from nut seeds? Explain. (2 marks)
(d) State one method that can be used ‘to convert liquid oil from nut seeds into solid. (1 mark)
(e) Describe how soap is manufactured from liquid oil from nut seeds. (3 marks)
(f) 0.44 g of an ester A reacts with 62.5 cm3 of 0.08 M potassium hydroxide giving an alcohol B and substance C. Given that one mole of the ester reacts with one mole of the alkali, calculate the relative molecular mass of the ester. (2 marks)
3 (a)Name the method that can be used to obtain pure iron (Ill) chloride from a mixture of iron (III) chloride and sodium chloride. (1 mark)
(b) A student was provided with a mixture of sunflower flour, common salt and a red dye. The characteristics of the three substances in the mixture are given in the table below.
The student was provided with ethanol and any other materials needed.
Describe how the student can separate the mixture into its three components. (3 marks)
(c) The diagram below shows part of a periodic table. The letters do not represent the actual symbols of elements. Use the diagram to answer the questions that follow.
(i) Explain why the oxidising power of W is more than that of X. (2 marks)
(ii) How do the melting points of R and T compare? Explain. (2 marks)
(iii) Select an element that could be used:
(I) in Weather balloons; (1 mark)
(II) for making a cooking pot. (l mark)
(d) (i) Classify the substances water, iodine, diamond and candle wax into elements and compounds. (2 marks)
(ii) Give one use of diamond. (1 mark)
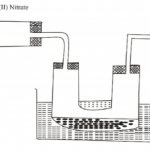
(a) The diagram below represents a dry cell. Use it to answer the questions that follow.
(i) Which of the letters represent:
(I) carbon electrode? (1 mark)
(II) the electrolyte? (1 mark)
(ii) One of the substances used in a dry cell is manganese (IV) oxide. State two roles of manganese (IV) oxide in the dry cell. (2 marks)
(b) Below is a simplified electrolytic cell used for purification of copper. Study it and answer the questions that follow.
(i) Identify the cathode. (1 mark)
(ii) Write the equation for the reaction at the anode. (1 mark)
(iii) What name is given to L? (1 mark)
(iv) A current of 0.6 A was passed through the electrolyte for 2 hours. Determine the amount of copper deposited. (Cu = 63.5; l Faraday = 96,500 coulombs). (3 marks)
(v) State two uses of copper metal. (1 mark)
5 The set-up below can be used to generate a gas without heating. This occurs when substance M reacts with solid N.
(a) (i) Complete the table below gluing the names of substance M and solid N if the gasses generated are chlorine and sulphur (IV) oxide. (2 marks)
(ii) Complete the diagram above to show how a dry sample of sulphur (IV) oxide can be collected. (3 marks)
(b) Describe two chemical methods that can be used to test the presence of sulphur (IV) oxide. (3 marks)
(c) Other than the manufacture of sulphuric (VI) acid, state two uses of sulphur (IV) oxide. (2 marks)
6 (a) Other than concentration, state two factors that determine the rate of a reaction.(2 marks)
(b) In an experiment to determine the rate of reaction, excess lambs of calcium carbonate were added to 2 M hydrochloric acid. The mass of calcium carbonate left was recorded after every 30 seconds. The results are shown in the table below.
(i) Write the equation for the reaction that took place. (1 mark)
(ii) On the grid provided, plot a graph of mass of calcium carbonate vertical axis against time. (3 marks)
(iii) Determine the rate of reaction at the 105th second. (3 marks)
(c) Why does the curve level off after some time? (1 mark)
(d) On the same grid, sketch a curve for the same reaction using 4 M hydrochloric acid and label the curve R. (2 marks)
7 (a)Naturally occurring magnesium consists of three isotopes. 78.6% 24Mg; 10% 25Mg and 26Mg. Calculate to one decimal place, the relative atomic mass of magnesium. (2 marks)
(b) When magnesium burns in air, it forms a white solid and a grey-green solid. When a few drops of water are added to the mixture, a gas that turns red litmus paper blue is evolved.
Identify the:
(i) White solid. (1 mark)
(ii) gas evolved and state its use.
(I) Name of gas. (1 mark)
(II) Use of the gas. (1 mark)
(c) Two different samples of water (I and II) were tested with soap solution. Sample II was further subjected to two other processes before adding soap. 20 cm3 of each sample of water was shaken with soap solution in a boiling tube until a permanent lather was obtained. The results are shown in the table below.
(i) Identify the water sample that had temporary hardness. Explain your answer.(2 marks)
(ii) Explain why the results for sample II are different after distilling but remain unchanged after filtering. (2 marks)
(m) State two disadvantages of using both water samples for domestic purposes. (2 marks)
CHEMISTRY
Paper 3
(PRACTICAL)
1. You are provided with:
– 2.0 g of substance A, labelled solid A.
– Solution B, 0.05 M hydrochloric acid.
– Methyl orange indicator.
You are required to determine the:
– solubility of substance A in water.
– relative formula mass of substance A.
PROCEDURE I
(i)Place 200 cm’ of. tap water in a 250 ml beaker and keep it for use in step (vi).
(ii)Place all of substance A in a dry boiling tube.
(iii)Using a burette, measure 10.0 cm3 of distilled water and add it to the substance A in the boiling tube.
(iv)While stirring the mixture in the boiling tube with a thermometer, warm the mixture using a Bunsen burner, until the temperature rises to 65°C. Stop warming the mixture.
(v)Allow it to cool while stirring with the thermometer.
(vi)When the temperature drops to 60°C, start the stop watch/clock, place the boiling tube in the beaker with tap water prepared in step (i) above.
(vii)Continue stirring and record the temperature of the mixture after two minutes, then thereafter record the temperature of the mixture after every one minute interval and complete table 1. Retain the mixture with the thermometer inside for use in procedure II below.
(4 marks)
On the grid provided, plot a graph of temperature (vertical -axis) against time. (3 marks)
(a) Using the graph, determine the temperature (Ts) when 2.0 g of substance A dissolves completely in 10.0 cm3 of distilled water. (1 mark)
(b) Calculate the solubility of substance A in grams per 100 g water at temperature, Ts. (2 marks)
PROCEDURE II
Using a funnel, transfer all the mixture obtained from Procedure I into a 250 ml volumetric flask. Rinse the boiling tube and the thermometer with about 20 cm3 of distilled water and add the rinses into the volumetric flask. Repeat the rinsing two more times. Add about 100 cm3 of distilled water to the volumetric flask. Shake until all the solid dissolves. Add more distilled water to the mark. Label this as solution A. Fill the burette with solution A. Using a pipette and pipette filler, place 25.0 cm3 of solution B, into a 250 ml conical flask. Add three (3) drops of the indicator provided and titrate using solution A. Record your readings in table 2 below. Repeat the titration two more times and complete the table.
(3 marks)
(a) Calculate the:
(i) average volume of solution A used. (1 mark)
(ii) number of moles of hydrochloric acid, solution B used. (l mark)
(b) Given that two moles of acid react with one mole of substance A, calculate:
(i) number of moles substance A used. (1 mark)
(ii) concentration of solution A in moles per litre; (1 mark)
(iii) concentration of solution A in g per litre; (l mark)
(iv) relative formula mass of substance A. (1 mark)
2. You are provided with solid C. Carry out the following tests and record your observations and inferences in the spaces provided. Place all the solid C in a boiling tube. Add about 15 cm3 of distilled Water and shake until all the solid dissolves. Use 2cm3 portions of the Sodium solution in a test tube, for each of the tests in (a), (b), (c), (d), (e) and (f).
3 You are provided with substance L. Carry out the following tests and record your observations and inferences in the spaces provided. Use about 2 cm3 portions of substance L in a test-tube for each of the tests, (a), (b), (c) and (d).




assist me with answers( marking scheme
)