KNEC KCSE Past Papers General Science 2015
KCSE Past Papers General Science 2015
SECTION A: BIOLOGY (34 marks)
Answer all the questions in this section in the spaces provided.
1 (a) What is meant by the following terms:
(i) habitat; (1 mark)
(ii) population? (1 mark)
(b) Explain one effect for each of the following air pollutants to the environment:
(i) sulphur (IV) oxide; (2 marks)
(ii) smoke. (2 marks)
2 (a) Explain the significance of the position of testis in humans. (2 marks)
(b) State two reasons why reproduction is important. (2 marks)
3 (a) Figure 1 illustrates a longitudinal section of a bean seed.
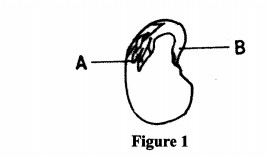
Name the parts labelled A and B. (1 mark) (b) (i) Distinguish between the terms epicotyl and hypocotyl. (1 mark)
(ii) Name a plant hormone that promotes apical dominance. (1 mark)
4 (a) State the meaning of the following terms:
(i) genetics; (1 mark)
(ii) genes. (1 mark)
(b) What is meant by:
(i) complete dominance; (1 mark)
(ii) phenotype? (1 mark)
5 (a) Explain why Lamarck’s evolution theory was discredited. (2 marks)
(b) What is meant by complete metamorphosis in insects? (1 mark)
6 State two similarities between the endocrine and the nervous system.(2 marks)
7 (a) How are the vascular bundles arranged in young:
(i) monocotyledonous stem; (1 mark)
(ii) dicotyledonous stem? (1 mark)
(b) Figure 2 illustrates the human arm.

Describe how the muscles work to move the forearm from point C to D. (1 mark)
8 (a) Name the causative agent of syphilis. (1 mark)
(b) Which hormone brings about ovulation in humans? (1 mark)
9 (a) State two ways in which genetic counselling is used in the community. (2 marks)
(b) State how the following eye defects can be corrected:
(i) short sightedness; (1 mark)
(ii) astigmatism. (1 mark)
10 State two differences between simple reflex action and conditioned reflex action. (2 marks)
SECTION B: CHEMISTRY (33 marks)
Answer all the questions in this section in the spaces provided.
11 (a) State the two conditions under which Graham’s Law of diffusion operates. (1 mark)
(b) Figure 3 shows a set-up in which the porous pot contained air.

(i) State the observation made in the U-tube if air was replaced with carbon (IV) oxide. (1 mark)
(ii) Explain the observation made in (b) (i) above. (1 mark)
12 (a) What is meant by the term molarity of 5 solution? (1 mark)
100 cm3 of O.l M sodium hydroxide solution was mixed with 100 cm3 of l M sodium 5 hydroxide solution.
(i) calculate the number of moles contained in the mixture; (2 marks)
(ii) Determine the molarity of the mixture. (2 marks)
13 (a) Complete the following table:

(2 marks)
(b) State two pollution effects of detergents to the environment. (2 marks)
14 Explain the following statements:
(a) sulphur (IV) oxide is a bleaching agent in wet conditions; (2 marks)
(b) newspapers tum yellow-brown after exposure to sunlight over- a period of time. (1 mark)
15 Figure 4 is a representation of Down’s cell used in the extraction of sodium metal. Study it and answer the questions that follow.
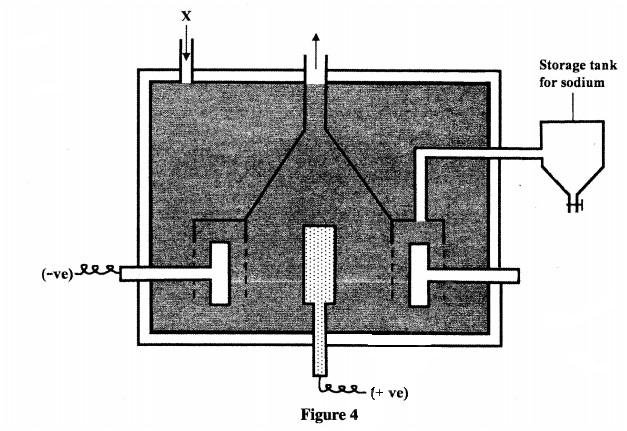
(a) Name the materials that are introduced at X. (1 mark)
(b) Write an equation for the reaction that occurs at the anode. (1 mark)
(c) What material is the cathode made of? (1 mark)
(d) Give two industrial uses of sodium metal. (1 mark)
16 (a) What is meant by “enthalpy of combustion”? (1 mark)
(b) The table below shows heating values of some fuels. Use it to answer the questions that follow

(i) Identify the best fuel for use in jet engines. (1 mark)
(ii) Give two reasons for the answer in b(i) above. (1 mark)
(iii) Which fuel has the greatest pollution effect? Explain. (2 marks)
17 Explain how each of the following affects rate of chemical reaction.
(a) Increase in surface area; (2 marks)
(b) Decrease in concentration. (2 marks)
18 (a) When chlorine gas is passed through a solution of potassium iodide, the following reaction takes place.
Identify the oxidising agent in the reaction. (1 mark)
(b) Explain the pollution effects of chloroflourocarbons (CFCs) to the environment. (2 marks)
19 When a green solid is heated, it forms a black residue and produces a colourless gas which forms a white precipitate with a solution of calcium hydroxide
(a) Identify the green solid. (l mark)
(b) Write an equation for the reaction that produces the white precipitate.(1 mark)
SECTION C: PHYSICS (33 marks)
Answer all the questions in this section in the spaces provided.
20 Figure 5 is a diagram of a periscope.
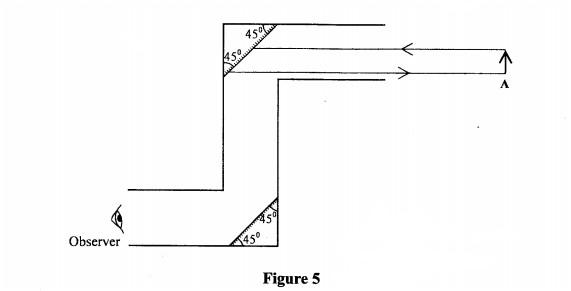
Complete the ray diagram to show the image of object A as seen by the observer.(3 marks)
21 An ebonite rod is charged by rubbing with fur. The rod becomes negatively charged while the fur becomes positively charged. Explain how this happens. (2 marks)
22 Draw and label a simple electric circuit diagram having a cell, a bulb, a switch and an ammeter. (Use circuit symbols) (2 marks)
23 State two factors that affect the speed of sound in air. (2 marks)
24 Describe a:
(a) transverse wave; (1 mark)
(b) longitudinal wave. (1 mark)
25 A student placed two iron nails in contact with one end of a bar magnet. The student observed that the free ends of nails moved apart from each other. Explain this observation.(3 marks)
26 Define electrical resistance and state its SI unit. (2 marks)
27 Figure 6 shows a set-up used to study the effect of placing a triangular glass prism in the path of white light

(a) State what will be observed on the screen. (1 mark)
(b) Name the phenomenon being studied. (1 mark)
28 A student made two heating coils using two different wires and used the coils to heat equal amounts of water in identical containers for the same length of time. At the end of heating, water in one of the containers was at a higher temperature than the other. State two factors that would have led to the difference in temperatures. (2 marks)
29 Figure 7 shows an object O placed infront of a converging lens Which has the principle focus at F. (3 marks)

On the figure, construct a ray diagram to locate the image formed.
30 A shopkeeper pays an electricity bill of ksh. 180.90 for using four identical bulbs for 3 hours per day for 30 days. If the cost of electricity is ksh. 6.70 per kilowatt hour, determine the power of each bulb. (3 marks)
31 State the role of the control grid in a cathode ray oscilloscope (CRO). (1 mark)
32 State any two dangers of exposure to X-rays. (2 marks)
33 Explain how a photographic film is used to detect radiations. (2 marks)
34 Explain how a P-N junction diode can be forward biased. (2 marks)