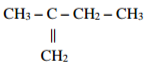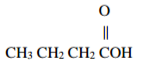KCSE Chemistry Paper 1 – 2015 KCSE Ikutha Sub-County Joint Examination
2015 KCSE Ikutha Sub-County Joint Examination
Chemistry Paper 1
The diagram below shows a set-up of apparatus used to separate immiscible liquids.
a) Name the parts labelled A and B. (1 mark)
A _________________________________________________________________________________
B _________________________________________________________________________________
b) State the function of the part labelled A . (1 mark)
___________________________________________________________________________________
___________________________________________________________________________________
c) State the property of the mixture that makes it suitable to be separated by the method above. (1 mark)
___________________________________________________________________________________
___________________________________________________________________________________
3 marks
2. Study the information in the table below and answer the questions that follow. The letters do not
represent the actual symbols of the elements
| Element | Atomic number | Melting point (℃) |
| L | 11 | 97.8 |
| M | 13 | 660 |
| R | 19 | 63.7 |
i) Write the formula of carbonate of R and M (1 mark)
___________________________________________________________________________________
___________________________________________________________________________________
ii) Describe how the carbonate of M can be obtained from a mixture of carbonate R and M. (2 marks)
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
iii) R is more reactive than L. Explain. (1 mark)
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
3 marks
In an experiment, concentrated sulphuric acid was put in a beaker and exposed to air for one week as
shown below.
i) What observation was made after one week? Explain. (2 marks)
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
ii) What property of sulphuric acid was being investigated in the experiment? (1 mark)
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
3 marks
a) Define the term solubility. (1 mark)
___________________________________________________________________________________
___________________________________________________________________________________
b) A form four student wanted to determine the solubility of potassium nitrate. He obtained the
following results.
Mass of evaporating dish =15.13g
Mass of evaporating dish and solution =36.51g
Mass of evaporating dish and salt =19.41g
Use the information above to calculate the solubility of potassium nitrate. (3 marks)
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
4 marks
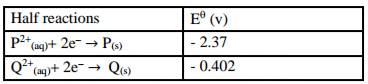
The table below shows the standard electrode potentials of two elements P and Q.
Half reactions E
i) Draw a well labelled diagram of a cell that could be constructed from the pair of elements. (2 marks)
ii) Calculate the e.m.f. of the cell above. (1 mark)
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
iii) Predict whether displacement reaction between metal Q and solution of P from e.m.f in 5 (ii) above will occur. (1 mark)
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
4 marks
The diagram below shows an incomplete set – up for the laboratory preparation and collection of
chlorine gas. Study it and answer the questions that follow.
a) Complete the set up to show how dry chlorine gas is collected. (2 marks)
b) Name substance Q. (1 mark)
___________________________________________________________________________________
3 marks
If aqueous lead (II) nitrate is added to aqueous solution potassium iodide, a bright yellow precipitate is
formed.
i) Write down the formula of the precipitate formed. (1 mark)
___________________________________________________________________________________
ii) Write the ionic equation for the reaction above. (1 mark)
___________________________________________________________________________________
___________________________________________________________________________________
2 marks
Zinc carbonate decomposes on heating producing a gaseous product and a residue. What volume of the
gaseous product at s.t.p. is produced from 2.5g of the carbonate?
(Zn = 65, C = 12, O = 16, MGV at s.t.p =22400cm3)
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
3 marks
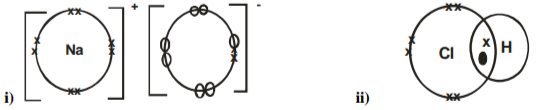
Identify the type of bond formed in (i) and (ii)
i) _________________________________________________________________________________
ii) _________________________________________________________________________________
2 marks
Give the systematic names of the following compounds.
a)
___________________________________________________________________________________
b)
___________________________________________________________________________________
c)
___________________________________________________________________________________
3 marks
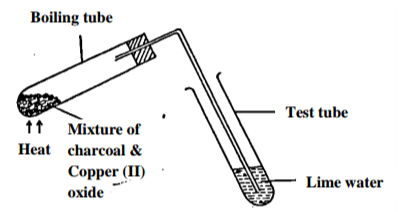
The set up below was used to investigate a chemical property of carbon. Study it and answer the
questions that follow
i) What observations were made on heating the mixture? (2 marks)
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
ii) What is the industrial application of carbon in terms of property investigated above? (1 mark)
___________________________________________________________________________________
___________________________________________________________________________________
3 marks
In an experiment, a few drops of concentrated nitric (V) acid were added to aqueous iron (II) sulphate in a test tube. Excess sodium hydroxide solution was then added to the mixture.
a) State the observations that were made when;
i) Concentrated nitric (V) acid was added to aqueous iron (II) sulphate. (1mark)
___________________________________________________________________________________
___________________________________________________________________________________
ii) Excess sodium hydroxide was added to the mixture. (1mark)
___________________________________________________________________________________
___________________________________________________________________________________
b) Write an ionic equation for the reaction that occurred in a) ii) above (1 mark)
___________________________________________________________________________________
___________________________________________________________________________________
3 marks
Consider the reaction represented by the equation:
N2 (g) + O2 (g) ⇌ 2NO (g) ∆H = + 1259kJ
Explain the effects of the following on the reaction;
a) An increase in pressure (1 mark)
___________________________________________________________________________________
___________________________________________________________________________________
b) Increase in temperature (2 marks)
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
3 marks
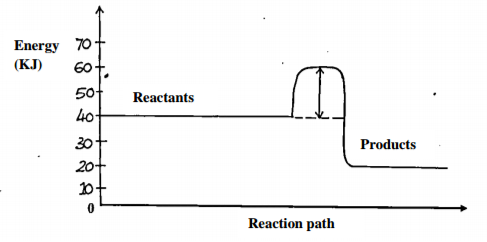
Study the energy level diagram below and answer the questions that follow.
i) State and explain whether the reaction represented is endothermic or exothermic. (1 mark)
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
ii) From the diagram, determine;
I. The activation energy (1 mark)
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
II. Enthalpy of reaction (1mark)
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
3 marks
Explain why when heating substances with non – luminous flame, tubes should not be placed very close to the top of the chimney. ___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
2 marks
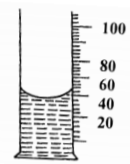
a) State graham’s law of diffusion. (1mark)
___________________________________________________________________________________
___________________________________________________________________________________
b) Two gases A and B diffuse from two opposite ends of the glass tube as shown. After 12 seconds
gas B was detected at point P and A was detected 4 seconds later.
Calculate the relative molecular mass of A given the relative molecular mass of B is 2. (3 marks)
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
4 marks
Starting with copper metal, describe how a sample of crystals of copper (II) chloride may be prepared in the laboratory.
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
3 marks
Thorium 
form the nuclide 
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
3 marks
Explain why the reaction between 1g of calcium carbonate and 1M hydrochloric acid is faster than the reaction between 1g of calcium carbonate and 1M butanoic acid.
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
2 marks
A hydrocarbon gas Y in which the percentage of hydrogen by mass is 14.3% occupies a volume of
2.24dm3 at s.t.p. and weighs 7g.
i) Determine the empirical formula of Y. (C = 12, H = 1.0) (2 marks)
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
ii) Give the structural molecular formula of Y. (2 marks)
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
4 marks
When magnesium was burnt in air, a solid mixture was formed. On addition of water to the mixture a
gas which turned moist red litmus paper blue was evolved. Explain these observations.
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
2 marks
In an experiment to prepare nitrogen (I) oxide, ammonium nitrate was gently heated in a flask.
a) State and explain how the gas was collected. (1 mark)
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
b) A sample of the gas was tested with damp blue and red litmus papers. What observations were made?
(1 mark)
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
2 marks
The following are standard electrode potentials of some half-cell reactions. Use the data to answer the questions that follow.
| Element | Electrode potentials |
| S | – 1.37 |
| T | – 0.83 |
| U | 0.00 |
| V | + 0.58 |
| W | + 1.46 |
i) Suggest the identity of element U. (1 mark)
___________________________________________________________________________________
ii) Which of the above elements is the most:
I) Reducing agent? Explain. (2 marks)
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
II) Oxidizing agent? Explain. (2 marks)
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
5 marks
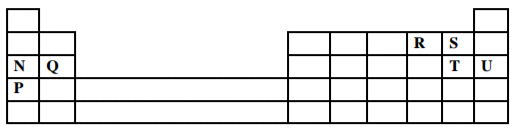
The grid below is part of the periodic table. Use it to answer the questions that follow.
(The letters do not represent the actual symbols of the elements)
a) Indicate in the grid the position of an element represented by letter V, Whose atomic number is 14.
(1 mark)
b) Select a letter which represents a mono atomic gas. (1 mark)
___________________________________________________________________________________
c) Write an equation for the reaction between Q and T. (1 mark)
___________________________________________________________________________________
___________________________________________________________________________________
3 marks
In an experiment, dilute hydrochloric acid was added to sodium hydroxide solution drop wise. The
concentration of sodium hydroxide was noted at regular time intervals.
i) Sketch a graph of concentration (y-axis) against time interval to show the concentration of sodium
hydroxide changes. (2 marks)
ii) Explain the shape of the curve sketched above. (1 mark)
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
3 marks
Below is a set-up of apparatus used to prepare hydrogen gas in the laboratory. Study it and answer the questions that follow.
a) Write the chemical equation for the two reactions taking place in the above set up. (1 mark)
___________________________________________________________________________________
___________________________________________________________________________________
b) State the chemical test for hydrogen gas. (1 mark)
___________________________________________________________________________________
___________________________________________________________________________________
Below is a table of 1st ionization energies for elements A, B, C and D which are metals.
| Elements | A | B | C | D |
| Ionization energies KJ/mol | 494 | 418 | 519 | 376 |
a) What is meant by 1st ionization energy? (1 mark)
___________________________________________________________________________________
___________________________________________________________________________________
b) With an explanation, arrange the elements in order of increasing reactivities. (1 mark)
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
2 marks
a) What are alkali metals? (1 mark)
___________________________________________________________________________________
___________________________________________________________________________________
b) Explain why potassium atom is larger than sodium atom. (1 mark)
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
2 marks