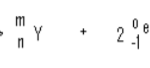KNEC KCSE Chemistry Paper 1 – 2014 Homa-Bay Mock
2014 Homa-Bay Mock
Chemistry Paper 1
State two reasons why most apparatus in the laboratory are made of glass.
2 marks
The following is an organic compound represented as CH3CH2COOCH2CH3
(i) Name the organic acid and alkanol used in making the compound (2mks)
(ii) Name the organic compound and the gas formed when the alkanol in (i) above is reacted
with Potassium (1mk)
3 marks
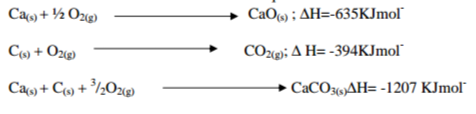
Use the information below to answer the question that follows
– Calculate the enthalpy change for the reaction.
3 marks
(a) What is the role of the following parts during fractional distillation of a mixture of water
and ethanol
(i) Fractionating column (1mk)
(ii) Glass beads in the fractionating column (1mk)
(b) State any one application of fractional distillation process (1mk)
3 marks
Name the process which takes place when:
(i) Iodine changes directly from solid to gas (1mk)
(ii) Fe2+(aq) changes to Fe3+(aq) (1mk)
(iii) White sugar changes to black solid when mixed with excess concentrated sulphuric (VI) acid
(1mk)
3 marks
The melting point of phosphorous trichloride is -91oC while that of sodium chloride is 801oC.
In terms of structure and bonding. Explain the difference in their melting point
3 marks
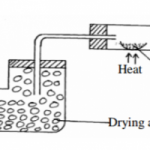
(a) Name a suitable drying agent to be used to dry chlorine gas (1mk)
(b) Chlorine reacts with red hot powder to give iron (III) chloride but not iron (II) chloride.
Explain? (1mk)
(c) Sodium hydroxide reacts with chlorine to form bleaching powder. Write a balanced equation
for the reaction (1mk)
3 marks
The electronic arrangement of elements are represented by letters A to D are as follows
A:2.8.6 B:2.8.2 C:2,8,1 D2:8.8
(a) Select the element which forms
(i)Double charged cation (1mk)
(ii) A soluble carbonate. (1mk)
(b) Which element has the shortest atomic radius? (1mk)
3 marks
Describe how a sample of Lead (II) chloride can be prepared using the following reagents dilute
nitric (V) acid; dilute hydrochloric acid and lead carbonate.
3 marks
A radioactive element of mass 50g has a half-life of 10 seconds.
(a) Sketch a graph of mass against time to show how the element mass varies with time (2mks)
(b) Give one use of radioactive in industries (1mk)
3 marks
State and explain one disadvantage of using hard water in boilers
2 marks
Hydrogen sulphide gas was passed through a solution of iron(III) chloride
(i) State and explain the observations made (2mks)
(ii) Write an ionic equation for the reaction taking place in (i) above (1mk)
3 marks
The apparatus below was set up to show the catalytic oxidation of ammonia. Study the diagram and
answer the questions that follow:
(i) Write an equation for the reaction that takes place in the gas jar (1mk)
(ii) What is the role of hot nichrome wire? (1mk)
(iii) Write the formula of the complex ion formed when excess ammonia gas is passed
through a solution containing Zn2+ ions. (1mk)
3 marks
A solution of silver nitrate was put in a container made of metal Q for 1 day. Given that:
Determine whether or not a reaction occurred between silver nitrate and metal Q (2mks)
2 marks
The table below shows the solubility of salt at various temperatures
| Temperature | Solubility g/100g of water |
| 0 | 36 |
| 40 | 30 |
| 80 | 25 |
| 110 | 20 |
What would happen if a sample of a saturated solution of the salt 40oC is heated to 80oC?
Explain.
2 marks
The equation given below represents a redox reaction
(i) Write the equation of the reduction process (1mk)
(ii) Which substances is oxidized? (1mk)
2 marks
When a current of 1.5 amperes was passed through cell containing M2+ ions on metal M for 15
minutes the mass of the cathode increased by 0.26g. (1F=96500C)
(i) Calculate the quantity of electricity used (1mk)
(ii) Determined the relative atomic mass of metal M (2mks)
3 marks
State any two differences between luminous and non-luminous flames.
2 marks
(a) State Graham’s law of diffusion (1mk)
(b) The molar masses of gas U and V are 16.0 and 44.0 respectively. If the rate of diffusion of
U through the porons materials is 12cm3-1. Calculate the rate of diffusion of V through the same materials (2mks)
3 marks
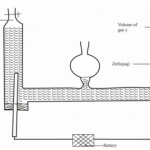
The set up below was used to collect a dry sample of a gas.
Give two reasons why the set-up cannot be used to collect carbon (IV) oxide gas.
2 marks
Dilute sulphuric acid does not react fully with calcium carbonate while dilute hydrochloric acid
reacts fully with calcium carbonate liberating carbon (IV) oxide. Explain.
2 marks
On complete combustion of 0.5g of a hydro carbon; 1.257g of carbon (IV) oxide and 0.514g of
water were produced. If the relative molecular mass of the hydrocarbon is 84, determine the
molecular formula (C=12,H=1,O=16).
3 marks
The conversion of SO2 to SO3 in the contact process is shown by the equation
(a) What would be the effect of?
(i) Increasing the concentration of Oxygen (1mk)
(ii) Increasing the temperature (1mk)
(b) Write an equation for the sulphuric (VI) acid from Oleum (1mk)
3 marks
Sulphur burns in air to form sulpur (IV) oxide. A simple energy level energy level diagram for the
reaction is given below. Study the diagram and answer the questions that follow:
(a) What do the following represents?H1 and H3 (2mks)
(b) Write an expression for H3in terms of H1and H2 (1mk)
3 marks
Given the reaction below
(a)State how the following factors affect the rate of reaction giving explanation (1mk)
(b) Using Zinc powder instead of granules (1mk)
(c) Heating the reactants (1mk)
3 marks
The flow chart below shows steps used in the extraction of zinc from one of its ores
(a) Name the process that is used in step 2 to concentrated (1mk)
(b) Write an equation for the reaction which takes place in step 3 (1mk)
(c) Name one use of zinc other galvanizing (1mk)
3 marks
The set up below used to obtain a sample of iron
(a) Identify the gas collected ( ½ mk)
(b) What observation is made on the excess iron (III) oxide? ( ½ mk)
(c) Write equations for the two reactions that take place in the combustion tube (2mks)
3 marks
The table below shows PH values of some solutions
| Solution | A | B | C | D |
| PH values | 13 | 7 | 1 | 6.5 |
(a) What solution reacts vigorously with Magnesium metal? (1mk)
(b) Which solution is likely to be that of Lemon juice? (1mk)
(c) Which solution forms complex ions with zinc (II) oxide? (1mk)
3 marks
When a few drops of aqueous ammonia were added to Copper (II) Nitrate solution a light blue
precipitate was formed. On addition of more aqueous ammonia a deep blue solution was formed.
Identify the substances responsible for the:
(a) Light blue precipitate (1mk)
(b) Deep blue solution (1mk)
2 marks
Explain why there is general increase in the first ionization energies of the elements in period 3 of
the periodic table from left to right.
2 marks