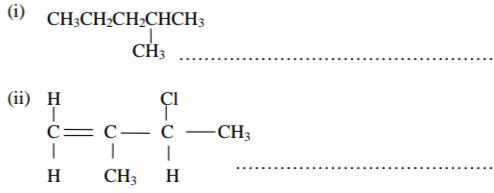KNEC KCSE Chemistry Paper 2 – 2014 Cross Country Mock
2014 Cross Country Mock
Chemistry Paper 2
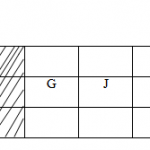
The figure below shows a section of the periodic table. The letters do not represent the
actual symbols of the elements
(a) Give the name of the family of the elements to which M belongs (1mk)
(b) Write the electronic configuration of the stable ion of element;
(i) L (1mk)
(ii) P (1mk)
(c) Give one use of element (1mk)
(i) A(1mk)
(ii) M (1mk)
(d) The melting point of element C is higher than that of G. explain (2mks)
(e) What is the structure of element J? (1mk)
(f) With explanation compare the atomic radius of O and P? (2mks)
(g) Which is the most reactive Non-metal? Explain (1½mks)
13 marks
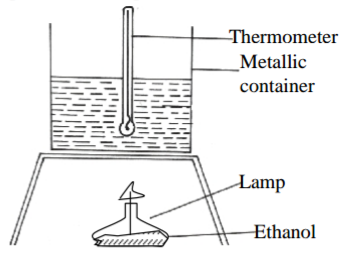
(a) State two factors that should be considered when choosing a fuel for cooking (2mks)
(b) The diagram below represents a set-up that was used to determine the molar heat of
combustion of ethanol.
During the experiment, the data given below were recorded;
Volume of eater 450cm3
Initial temperature of water 25oC
Final temperature of water 46.5oC
Mass of Ethanol + lamp 125.5g
Mass of Ethanol + lamp 124.0g
After burning
Calculate the ;
(i) Heat evolved during the experiment (Density of eater = 1g/cm3) specific heat capacity of water =4.2KJ/Kg/K) (3mks)
(ii) Molar heat of combustion of ethanol (2mks)
(iii) Write the thermo-chemical equation for the combustion of ethanol (1mk)
(iv) Molar heat of combustion of ethanol obtained in b(i) above lower than the theoretical value.
State the two sources of error in the experiment. (C=12 O=16 H=1) (2mks)
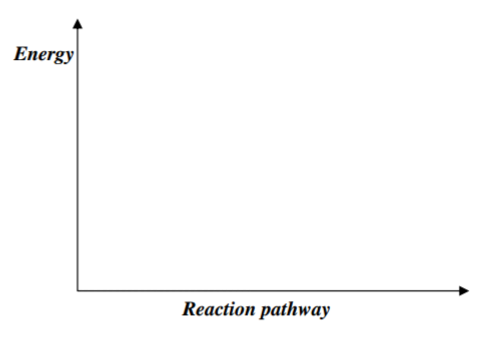
(d) On the axis, draw an energy level diagram for the combustion of ethanol. (2mks)
12 marks
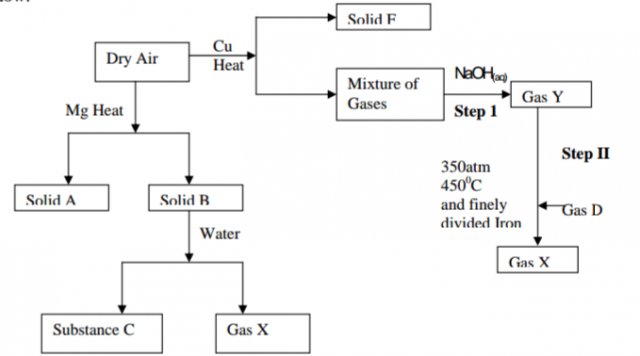
Study the flow chart below that shows some reactions of dried air then answer the questions
that follow:
(a) Identify; (2mks)
(i) Solid A
(ii) Gas D
(iii) Gas X
(iv) Solid B
(b) Whys is the amount of solid B obtained much less than solid A? (2mks)
(c) Write a balanced equation for the reaction between solid B and water (2mks)
(d) (i) How can gas Y be obtained from gas X in the laboratory? (2mks)
(ii) Write an equation for the process in d(i) above (1mk)
(e) Which gas is absorbed by NaOH in step I (1mk)
(f) Gas Y in step I is impure. Name one impurity it contains. (1mk)
(g) (i) What name is given to the process that occurs in step II? (1mk)
(ii) Explain the effect of using much lower temperature than that in step II (2mks)
13 marks
The table below gives the volume of gas produced when different volume 1M hydrochloric acid was
reacted with 1.6g of zinc powder at room temperature.
| Volume of 1M HCl (cm3) | Volume of gas (Cm3) |
| 0 20 40 60 80 100 |
0 200 400 600 600 600 |
(a)Write an equation for the reaction between zinc and HCl (1mk)
(b) Plot a graph of the gas produced against the volume of the acid added. (3mks)
(c) From the graph determine;
(i) The volume of gas produced if 25cm3 of 1M HCl had been used. (1mk)
(ii) The volume of 1M HCl which reacted completely with 1.6g of zinc powder (1mk)
(d) State and explain the effect on the rate of production of the gas if;
(i) 1.6g of zinc granules were used instead of zinc powder. (2mks)
(ii) 2M HCl was used instead of 1M HCl (2mks)
10 marks
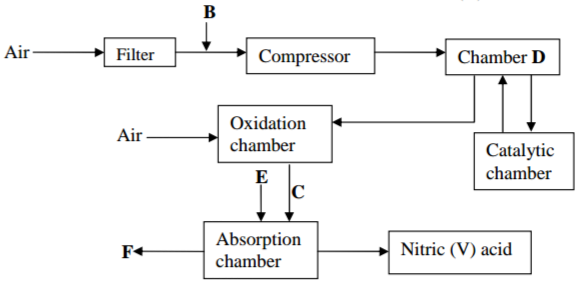
The flow chart below shows the industrial manufacture of Nitric (V) acid
(a) Identify substances B, C, E and F; (2mks)
(b) Describe what happens in the catalytic chamber (2mks)
(c) State what takes place in chamber D (1mk)
(d) 60-65% Nitric (V) acid is produced in the absorption chamber. Describe how the acid
can be concentrated (2mks)
(e) Copper reacts with Nitric (V) acid and not hydrochloric acid. Explain (2mks)
10 marks
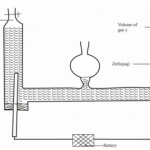
Study the given reduction potentials and answer the questions that follow.
(The letters do not represent actual symbols of the elements)
(a) (i) Which element is likely to be hydrogen? (1mk)
(ii) Draw an electrochemical cell made up of Y and X half cells. Show the direction
of flow of electrons (2mks)
(iii) Draw a diagram of a set-up showing how a spoon made of iron can be coated with silver metal
(2mks)
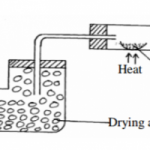
(b) The diagram below represents a set-up that was used during electrolysis of a solution of magnesium sulphate using inert electrodes.
(i) Identify the ions present in the electrolyte (2mks)
(ii) Write ionic equations for the reactions occurring at; (2mks)
Cathode
Anode
(iii) Calculate the quantity of electricity in coulombs that will liberate 1.2dm3 of oxygen gas at r.t.p
(Molar gas volume at r.t.p = 24dm3) (1 Farady = 95600C) (3mks)
12 marks
(a) Give the systematic names of the following compounds. (2mks)
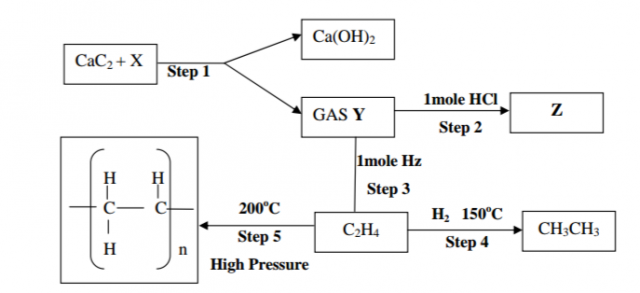
(b) Study the flow chart below and answer the questions that follow;
(i) Identify reagent X (1mk)
(ii) Name the catalyst used in step 4 (1mk)
(iii) Draw the structure of gas Y (1mk)
(iv) What name is given to the process that takes place in step 5 (1mk)
(v) Identify substance Z (1mk)
(vi) Draw the structure of substance Z (1mk)
(vii) State any two environmental effects of the product in step 5 (2mks)
10 marks