KNEC KCSE Chemistry Paper 2 – 2014 Homa-Bay Mock
2014 Homa-Bay Mock
Chemistry Paper 2
(a) The grid given below represents part of the periodic table. Study it and answer the questions that follow.
(The letters do not represent the actual symbols of elements )

(i) Giving reasons, select the element which is
I. Most reactive non metal (2mks) ………………………………………………………………………………………………………………
………………………………………………………………………………………………………………
II. Most reactive metal (2mks) ………………………………………………………………………………………………………………
………………………………………………………………………………………………………………
(ii) How does reactivity of A compare with that of B. Explain (1mk) ………………………………………………………………………………………………………………
………………………………………………………………………………………………………………
(iii) Explain why the atomic radius of K is smaller than that of G (1mk) ………………………………………………………………………………………………………………
………………………………………………………………………………………………………………
(iv) An element W forms ion W2-, if w is in period 3, indicate the position of W on the grid (1mk) ………………………………………………………………………………………………………………
(v) Write the formula of the compound formed when C reacts with H (1mk) ………………………………………………………………………………………………………………
(b) Study the information in the table below and answer the questions that follow

(i) Name two substances which are gaseous at room temperature (1mk)
………………………………………………………………………………………………………………
………………………………………………………………………………………………………………
(ii) Select the substance that could be dissolved in water and be separated from the solution by
Fractional distillation (1mk) ………………………………………………………………………………………………………………
(iii) Which substance could be an electrolyte? (1mk) ………………………………………………………………………………………………………………
(iv) Element U has low M.P and B.P whereas W has high M.P and B.P. Explain (2mks) ………………………………………………………………………………………………………………
13 marks
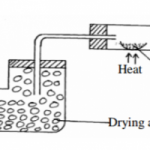
(a)The diagram below shows part of the processes in the manufacture of Nitric (V) acid
(i) What is the work of the purifier (1mk) ………………………………………………………………………………………………………………
(ii) State the pressure used in the compressor (1mk) ………………………………………………………………………………………………………………
(iii) State two functions of the heat exchanger (1mk) ………………………………………………………………………………………………………………
………………………………………………………………………………………………………………
(iv) Name the catalyst used in the catalytic chamber (1mk) ………………………………………………………………………………………………………………
(v) Write equation of the reaction that takes place in:
(I) Catalytic chamber (1mk) ………………………………………………………………………………………………………………
(II) Reaction chamber (1mk) ………………………………………………………………………………………………………………
(III) Absorption tower (1mk) ………………………………………………………………………………………………………………
(b) (i) Calculate the volume of Oxygen that would be obtained from the decomposition of 21.25g of
Sodium Nitrate at s.t.p (1 mole of a gas occupies 22.4dm3 at stp, N=14, Na=23,O=10) (3mks)
(c) Name two commercial uses of Nitric (V) acid (2mks) …………………………………………………………………………………………………………
…………………………………………………………………………………………………………
12 marks
(a) The diagram below shows a set-up used to determine the standard electrode potential (E ) of Zinc
(i) I. Label parts A and B
A………………………………………….. (1mk)
B………………………………………….. (1mk)
II. Identify substance C
C……………………………………….
(ii) Write the equations of the reactions that take place at the electrodes (2mks)
Anode : ………………………………………………………………………………………………………………
Cathode : ………………………………………………………………………………………………………………
(b) Study the standard electrode potentials given below and answer the questions that follow.
The letters do not represent the actual symbols of the elements
(i) Which is the
I. Strongest reducing agent. Explain (1mk) ………………………………………………………………………………………………………………
II. Strongest oxidizing agent. Explain (1mk) ………………………………………………………………………………………………………………
(ii) Calculate the e.m.f of a cell made by metals S and V (1mk) ………………………………………………………………………………………………………………
(c) During electrolysis of an aqueous solution of a salt of metal Q, a current of 2.0A was passed
for 32 minutes and 10 seconds. The mass of metal Q deposited was 2.24g
(1 Faraday=96500c, RAM of Q=112)
(i) Calculate the quantity of electricity passed (2mks)
(ii) Calculate the charge carried on the ion of metal Q (2mks)
11 marks
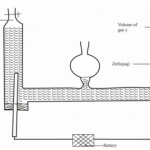
(a) In an experiment to study the rate of a reaction,2.0g of Manganese (IV) oxide was added to
100cm3 of hydrogen peroxide solution at 25oC. The volume of oxygen released was measured at 10
seconds intervals. The results obtained are tabulated below
| Time (sec) | 0 | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 |
| Volume (cm3) | 0 | 60 | 90 | 105 | 112 | 116 | 118 | 120 | 120 | 120 |
(i)Plot a graph of volume of gas (vertical axis) against time and label it X (3mks)
(ii) Use the graph to find:
(I) The volume of gas produced after 25 seconds (1mk) ………………………………………………………………………………………………………………
………………………………………………………………………………………………………………
(II) The time taken to produce 80cm3 of oxygen (1mk) ………………………………………………………………………………………………………………
………………………………………………………………………………………………………………
(iii) Explain why the volume of oxygen produced does not exceed 120cm3 (1mk) ………………………………………………………………………………………………………………
………………………………………………………………………………………………………………
(iv) Sketch a graph Y, on the same grid to show the results if the experiment was repeated
using hydrogen peroxide at 10oC. Explain (2mks) ………………………………………………………………………………………………………………
………………………………………………………………………………………………………………
(v) The mass of the solid residue after the experiment was found to be 2.0g when dried. Explain
(1mk) ………………………………………………………………………………………………………………
………………………………………………………………………………………………………………
9 marks
(a) The figure shows the extraction of Aluminium from bauxite
(i) Write the formula of bauxite (1mk) ………………………………………………………………………………………………………………
(ii) How is the ore (bauxite) concentrated before it is electrolysed (1mk) ………………………………………………………………………………………………………………
………………………………………………………………………………………………………………
(iii) Identify;
(I) Product A (1mk) ………………………………………………………………………………………………………………
(II) Electrolyte B (1mk) ………………………………………………………………………………………………………………
(III) Material used to make electrode C (1mk) ………………………………………………………………………………………………………………
(b) What is the purpose of dissolving electrolyte B in molten cyrolite (Na3AlF6) (1mk) ………………………………………………………………………………………………………………
………………………………………………………………………………………………………………
(c) Explain why anode has to be replaced from time to time (1mk) ………………………………………………………………………………………………………………
(d) Write the reaction for the chemical reaction that take place when aluminium reacts with Iron (III)
Oxide (1mk) ………………………………………………………………………………………………………………
(e) State any two uses of Aluminium (2mks) ………………………………………………………………………………………………………………
………………………………………………………………………………………………………………
10 marks
(a) Differentiate between lattice energy and hydration energy (2mks) ………………………………………………………………………………………………………………
………………………………………………………………………………………………………………
(b) Use the values given in the table below to answer the questions that follow
Given that lattice energy of MgCl2 is -2489 KJ/Mol
(i) Draw an energy cycle diagram for dissolving Magnesium in water (3mks)
(ii) Use your energy cycle diagram above to calculate the enthalpy of solution of Magnesium
chloride (2mks)
(b) (i) Define fuel (1mk) ………………………………………………………………………………………………………………
………………………………………………………………………………………………………………
(ii) Given that heat of combustion of S is 296.8KJ/Mol, Determine the heating value of S(S=32)
(2mks)
10 marks
(a) Give the systematic names for following compounds;
(i) CH3CH2CH2CH2OH (1mk) ………………………………………………………………………………………………………………
(ii) 
(iii) CH3CH2CH3 (1mk) ………………………………………………………………………………………………………………
(b) Study the flow chart below and use it to answer the question that follow
(i) Name:
Substance A (1mk) ………………………………………………………………………………………………………………
Process I (1mk) ………………………………………………………………………………………………………………
Substance B (1mk) ………………………………………………………………………………………………………………
Gas C (1mk) ………………………………………………………………………………………………………………
Substance D (1mk) ………………………………………………………………………………………………………………
Compound E (1mk) ………………………………………………………………………………………………………………
(ii) Identify the type of Polymerization that results to the formation of compound E (1mk) ………………………………………………………………………………………………………………
(iii)If one mole of sugar, C6H12O6 produces two molecules of pure ethanol, C2H5OH
and two moles of carbon (IV) oxide gas as the only product;
I. Write an equation for the reaction (1mk) ………………………………………………………………………………………………………………
II. If 144kg of sugar (C6H12O6) was used to produce ethanol in this process, calculate the mass
in kg of ethanol produced (C=12,H=1, O=16) (3mks)
15 marks