KNEC KCSE Chemistry Paper 2 Question Paper / 2014 Kakamega County Mock
2014 Kakamega County Mock
Chemistry Paper 2
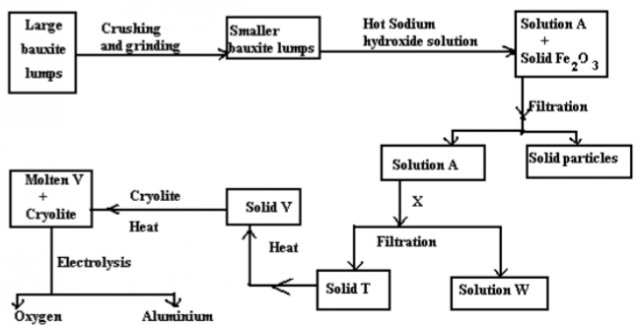
The flow diagram below shows the processes of purification of an aluminium ore and extraction of aluminium from it.
a. State why bauxite is first crushed and ground into smaller particles? (½mk) ______________________________________________________________________________________
b. Only aluminium oxide and silicon (IV) oxide dissolves chemically in the hot concentrated sodium
hydroxide to form solution A, Iron (III) oxide does not.
What property make aluminium oxide and silicon (IV) oxide react with sodium hydroxide solution?
I Silicon (IV) oxide_______________________________________________________________ (1mk)
II Aluminium oxide_______________________________________________________________ (1mk)
c. Write ionic equation for the reaction between sodium hydroxide solution and
I Silicon (IV) oxide________________________________________________________________ (1mk)
II Aluminium oxide_______________________________________________________________ (1mk)
d. I. Identify solid T___________________________________________________________________(1mk)
II Name substance X that could be used to precipitate out solid T from solution Q. (1mk) _________________________________________________________________________________
III Write equation for the formation of solid V from solid T (1mk) _____________________________________________________________________________________
e. Why is cryolite added to substance V? (½mk) _________________________________________________________________________________
f. Write half equation for the formation of aluminium at the cathode during electrolysis (1mk) ___________________________________________________________________________________
g. Give one advantage of locating aluminium extraction plant near the
I. Source of hydroelectricity_________________________________________________
__________________________________________________________________________(1mk)
II. Aluminium deposits or sea port._______________________________________________ (1mk)
h. Explain why an alloy of aluminium, instead of aluminium is used in overhead electric power cables. (1mk) ______________________________________________________________________________________
i. State why the ore for extracting aluminium is known as bauxite and not aluminium oxide (1mk) _____________________________________________________________________________________
13 marks
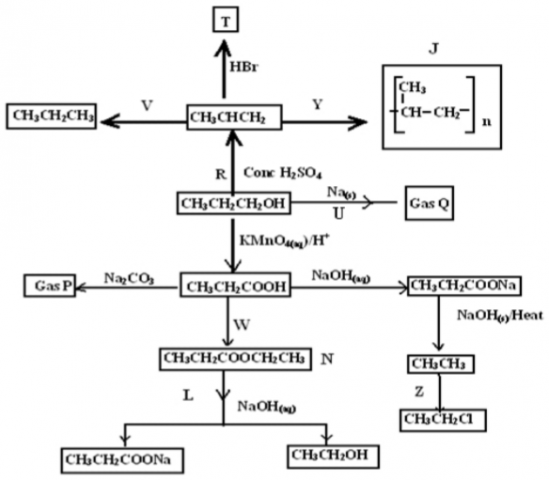
Study the reaction scheme below and answer the questions that follow.
a. Name I gas P_______________________________________________________
II gas Q_______________________________________________________
III Substance J________________________________________________ (1½mks)
b. Give the most probable structural formula of product T (1mk) _________________________________________________________________________________________
_________________________________________________________________________________________
_________________________________________________________________________________________
_________________________________________________________________________________________
c. I. Name reaction producing substance labeled N (1mk)
_____________________________________________________________________________________
II. State the characteristic property of N (1mk) ______________________________________________________________________________
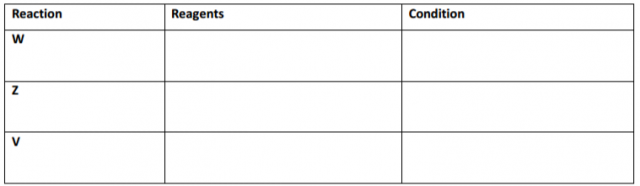
d. Complete the table below.
e. I. Name the organic product of reaction U (½mk) ________________________________________________________________________________________
II Write the equation for the reaction represented by U (1mk) ________________________________________________________________________________________
f. What is the specific name of process Y? (½mk) _________________________________________________________________________________________
g. What is the type of reaction represented by Z? (½mk) ________________________________________________________________________________________
h. Give two reasons why ethanoic acid has a higher melting point than ethanol when both of them have two carbon atoms. (2mks) _________________________________________________________________________________________
_________________________________________________________________________________________
9 marks
I. The following table gives the standard electrode potential for a number of half reactions.
| Half equation | Ee/volts |
| Zn2+(aq) + 2e– → Zn(s) | -0.76 |
| Fe2+(aq) + 2e– →Fe(s) | -0.44 |
| I2 (l) + 2e– →2I-(aq) | +0.54 |
| Fe3+(aq) + e– →Fe2+(aq) | +0.77 |
| Ce4+(aq) + e– →Ce3+(aq) | +1.61 |
a) Which half equation is used as the standard for the electrode potentials? (1mk) _______________________________________________________________________________________
b) From the table identify:
i. Strongest oxidizing agent_________________________________________________________(½mk)
ii. Strongest reducing agent_________________________________________________________(½mk)
(c) Identify two substances from the table which could be used to convert iodide ions to iodine. (1mks) ________________________________________________________________________________________
d. A half cell (I)is constructed by putting platinum electrode in a solution of 1M with respect to Fe2+ and Fe3+ ions.
The half cell is then connected to another half cell (II) with Fe2+ ions.
i. What is the e.m.f of this cell (1mk) _________________________________________________________________________________________
ii. Write equation for reactions taking place in each cell. (2mks)
(I)_______________________________________________________________________________________
(II) ______________________________________________________________________________________
iii. In which direction do electrons flow in the circuit? (½mk) _______________________________________________________________________________________
II. When copper (II) sulphate solution is electrolysed using platinum electrodes, the equation for the overall changes can be written as:
2CuSO4 (aq) + 2H2O(l) 2Cu(s) + O2 (g) + 2H2SO4 (aq)
a) State the observation made at
i. Anode________________________________________________________________________(½mk)
ii. Cathode______________________________________________________________________(½mk)
iii. In the solution_________________________________________________________________ (½mk)
b) Write ionic half equation for the reaction occurring at the anode. (1mk) _________________________________________________________________________________________
c) During electrolysis, 1.27g of copper is produced. Calculate
i. The volume of oxygen, measured at room temperature that would be produced in the same time.
(Cu=63.5, MGV= 24dm3, IF= 96 (2mks) __________________________________________________________________________________
__________________________________________________________________________________
__________________________________________________________________________________
__________________________________________________________________________________
__________________________________________________________________________________
ii. The time for which a current of 0.5A would be maintained to form these products. (1mk) __________________________________________________________________________________
__________________________________________________________________________________
__________________________________________________________________________________
12 marks
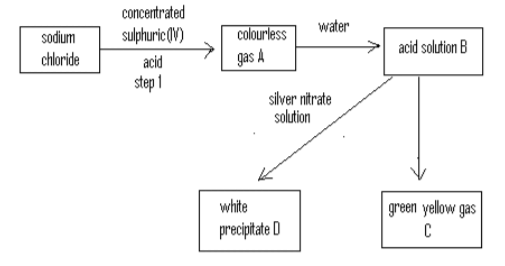
I. The diagram below summarizes the results of a series of chemical reactions.
a) Name: A___________________________________________________________________________(2mks)
B____________________________________________________________________________ C____________________________________________________________________________ D____________________________________________________________________________
b) What reagent would you use to convert B into C? (1mk) _____________________________________________________________________________________
c) Write equation for the reaction in step 1 (1mk) _______________________________________________________________________________________
d) Chlorine is used as bleaching agent. Name another substance that must be present for bleaching to occur. ___________________________________________________________________________________ (½mk)
e) Give one other use of chlorine. (1mk) _______________________________________________________________________________________
f) Explain the observation made when white precipitate D is exposed to sunlight. (2mks) ______________________________________________________________________________________
II One stage in the manufacture of nitric acid involves the oxidation of ammonia by the reaction:
4NH3 (g) + 5O2 (g) →4NO(g) + 6H2O (l)
a. State one condition under which this reaction is carried out. (1mk) _________________________________________________________________________________________
_________________________________________________________________________________________
b. Below is equation for the Haber process for the manufacture of ammonia.
i. Name one material which is available in large scale and can be used as a source of hydrogen for this process. _____________________________________________________________________(1mk)
ii. Give one reason why the reaction of nitrogen with hydrogen cannot be used to produce ammonia at
room temperature and pressure. (1mk) _________________________________________________________________________________
iii. To maintain a constant temperature, the bed of catalyst particles must be cooled. Why would the
temperature of the catalyst rise if it were not cooled? (½mk) __________________________________________________________________________________
iv. The iron catalyst used is fed in form of Fe3O4, but this is converted to minute crystals of iron when it is exposed to the hot mixture of nitrogen and hydrogen gases. Write an equation for the reaction
which converts the iron oxide to iron. (1mk) __________________________________________________________________________________
v. Suggest one disadvantage of using pure ammonia as fertilizer. (1mk) __________________________________________________________________________________
13 marks
I. The equation below is for the manufacture of ethanol by direct hydration of ethane.
H2O(g) +C2H4(g) C2H3OH(g) H= -46kJmol-1
a) State the effect an increased in pressure will have on the equilibrium yield of ethanol and give a reason for your answer. (2mks) _________________________________________________________________________________________
_________________________________________________________________________________________
b) At high pressure, addition polymerization of one of the compounds in the reaction mixture may occur. Give the name of the polymer produced and structure of the repeating unit. (2mks) _________________________________________________________________________________________
_________________________________________________________________________________________
_________________________________________________________________________________________
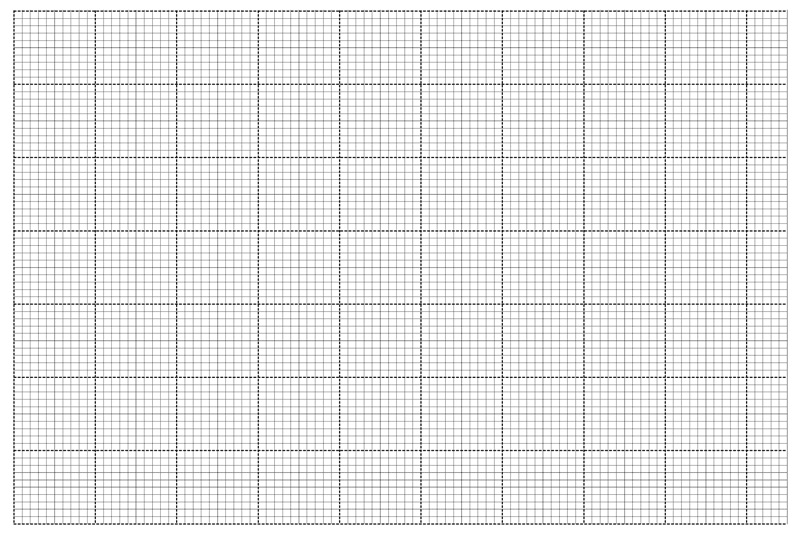
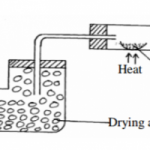
II. In an experiment, excess solid calcium carbonate was added to 100cm3 of 0.2M hydrochloric acid at 20oC .
The volume of carbon (IV) oxide produced was measured at regular time interval. The results of the
experiment are as shown in the table below.
| Time (seconds) | 0 | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 | 100 |
| Volume of carbon(IV)oxide(cm3) |
0 | 18 | 30 | 40 | 48 | 53 | 57 | 58 | 58 | 58 | 58 |
a) Draw a diagram of suitable apparatus for the experiment. (2mks) _________________________________________________________________________________________
_________________________________________________________________________________________
_________________________________________________________________________________________
_________________________________________________________________________________________
_________________________________________________________________________________________
b) On the grid provided plot of volume of CO2 produced against time. (3mks)
c) On the grid plot a graph you would expect if the acid was kept at 30oC. (1mk)
d) What is the rate of reaction if the volume of hydrogen per minute between 4th and 5th second (1mk) _________________________________________________________________________________________
_________________________________________________________________________________________
e) Other than raising the temperature state two ways by which the rate of reaction would be increased. (2mks) _________________________________________________________________________________________
_________________________________________________________________________________________
9 marks
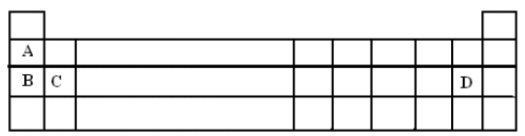
Below is a section of the periodic table. Use it for the questions below.
a. In the grid, place
i. M in the space with the most reactive non metal. (1mk).
ii R in the space which could be occupied by an element capable of forming a covalent compound RD3 (1mk)
b). Explain how the reducing power changes from A to B (2mks) _________________________________________________________________________________________
_________________________________________________________________________________________
c). How many protons are there in the atom of element C? __________________________________ (1mk)
d. Element B reacts with water.
i. State two observations you would expect to make when a small piece of B is added to water. (2mks) _________________________________________________________________________________________
_________________________________________________________________________________________
ii. Name the aqueous product formed in the above reaction and write chemical equation for the reaction. (2mks)
Product__________________________________________________________________________________
Equation_________________________________________________________________________________
9 marks
a. I. Aluminium chloride exists as a dimeric molecule Al2Cl6. Draw its dimeric structure. (2mks) _________________________________________________________________________________________
_________________________________________________________________________________________
_________________________________________________________________________________________
_________________________________________________________________________________________
_________________________________________________________________________________________
II. State why aluminium chloride exist as a dimer. (1mk)
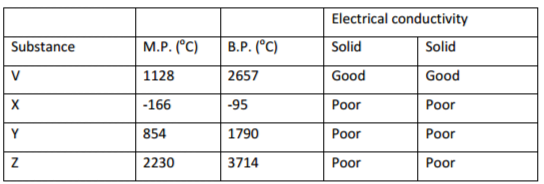
b. The table below gives some properties of four substances. Use it to answer the questions that follow.
i. Which substance has
Giant atomic structure ______________________________________________________________(1mk)
Giant metallic structure______________________________________________________________(1mk)
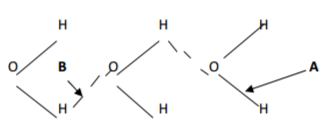
ii. The structure of water molecule can be represented as shown below.
Name the type of bonds represented by letters A_____________________________________(½mk)
B_____________________________________(½mk)
C Explain why sodium chloride conducts electricity when molten but not when solid. (2mks) __________________________________________________________________________________
__________________________________________________________________________________
8 marks
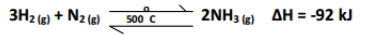

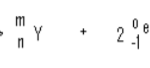



my fellow students chemestry is easy to pass