KNEC KCSE Chemistry Paper 2 Question Paper / 2015 KCSE Kirinyaga West
2015 KCSE Kirinyaga West
Chemistry Paper 2
a) An atom B (not actual symbol) can be represented as
(i) What does 80 stand for ?
ii) How many neutrons does it have?
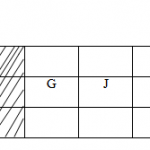
b) The grid below represents part of the periodic table. The letters are not actual symbols. Use it to answer
the questions that follows.
J K D M
W Q P
i) Explain the difference in atomic radii of:
I. K and W II. J and K
ii) Compare the reactivity of the following. Explain your answer.
I. M and Q II K and W
c) Study the information in the table below and answer the questions that follow. The letters do not
represent actual symbols.
Element Electron arrangement Atomic Ionic
of stable ion radius (nm) radius (nm)
A 2.8.8 0.197 0.099
B 2.8.8 0.99 0.181
C 2.8 0.16 0.065
D 2.8 0.186 0.095
E 2 0.152 0.068
F 2.8 0.072 0.136
i) Identify the elements that belong to the third period o§f the periodic table. (1mk)
ii) Arrange the elements you have identified in (i) above as they follow each other in the third period. (1mk)
iii) Is element F a metal or a non-metal. Explain your answer. (2mks)
4 marks
Study the flow diagram shown below.
a) Identify:
i) Metal ions in solution F (1mk)
ii) White precipitate K (1mk)
b) Write the formula of the ions in solution P. (1mk)
c) Explain how anions in solution of Q could be proved/tested in laboratory to be sulphate. (3mks)
d) What is double decomposition? (1mk)
e) Describe how you would prepare crystals of sodium nitrate starting with 200cm3 of 2M nitric (V) acid.
(3mks)
7 marks
Below is a diagram shown how hydrogen can be prepared in the laboratory and the study of the reducing action of hydrogen.
Copper (II) oxide
To suction pump
Liquid S
Cold
water
a) Define the term reduction as per the diagram above. (1mk)
b) Identify apparatus Q
c) Identify two mistakes in the set up.
d) Suggest a suitable drying agent K.
e) What is liquid M.
f) Explain the chemical reaction taking place in apparatus Q. (2mks)
g)(i) Name liquids (1mk)
ii) Give two chemical tests for liquid S (2mks)
b) State two uses of hydrogen gas. (2mks)
12 marks
The graph below shows the changes in conductivity when
a) 50cm3 of 0.1M nitric (V) acid is titrated with potassium hydroxide solution and
b) when 50cm3 of 0.1M methanoic acid is reacted with the same potassium hydroxide solution.
i) Which curve is represented by:
Curve Y (2mks)
Curve Z (2 Mks)
ii) Explain the changes in conductivity in regions:
AB (2mks)
BC (2mks)
iii) Why do the reaction (a) and (b) not give one curve and both are on neutralization reaction? (3mks)
iv) 50cm3 of 0.1M methanoic acid was reacted with 20cm3 of a solution of sodium carbonate of unknown concentration work out the concentration of the sodium carbonate. (2mks)
14 marks
a) Give the systematic names of the following compounds
i)CH2CHCH3 (1mk)
CH3
ii) CH3CH2CH2CCH (1mk)
b) State the observations made when Propan-1-ol reacts with:
i) Acidified potassium dichromate (VI) solution. (1mk)
ii) Sodium metal (1mk)
c) Ethanol obtained from glucose can be converted to ethene as shown below;
Step I Step II
C2H5OH CH2CH2
Name and describe the processes that take place in steps I and II
Step I
Step II (11/2mks)
d) Compounds M and N have the same molecular formula C3H6O2, compound M liberates carbon (VI)oxide on addition of aqueous sodium carbonate while compound N does not.Compound N has a pleasant smell. Draw the possible structures of: M,N
6 marks
a) i) State the Le Chatelier’s principle. (1mk)
ii) Carbon (II) oxide gas reacts with stream according to the equation.
H2(g) + CO2(g)
What would be effect of increasing the pressure of the system at equilibrium? Explain. (2mks)
ii) When the reaction in (ii) above was carried out at lower temperature, the yields of hydrogen and carbon
(IV) oxide increased. What is the sign of H for the reaction? Explain. (2mks)
b) The table below gives the volumes of oxygen gas produced at different times when hydrogen peroxide
decomposed in the presence of a catalyst.
Time (sec) 0 10 20 30 40 50 60
Vol.of O2 cm3 0 66 98 110 119 120 120
i) Name the catalyst used in this reaction. (1mk)
ii) On the grid provided, draw the graph of volume of oxygen gas produced (vertical) axis against time.
(3mks)
iii) Using the graph determine the rate of decomposition of hydrogen peroxide after 24 seconds.(2mks)
(Iv) Give a reason why the total volume of oxygen produced after 50 seconds remains constant. (1mk)
7 marks
a) The flow chart below shows properties of two allotropes of element Q.
i) Identify the allotropes
D (1mk)
B (1mk)
ii) Name element Q (1mk)
iii) Write a chemical equation for the reaction forming product P. (1mk)
iv) What term is given to the temperature of 960C shown above? (1mk)
(b)
(i) Name substance: X and Y ( 2 mks)
ii) What is the role of the following substances ?
a) Solid V (1 mk)
b)Fused calcium chloride (1mk)
c) Salt in the Ice + salt mixture (1mk)
iii) Explain why the fume chamber is used? (1mk)
iv) Write an equation for the reaction that took place in the combustion tube. (1mk)
7 marks