KNEC KCSE Chemistry Paper 1 – 2015 – Machakos County Trial
2015 KCSE Machakos County Trial
Chemistry Paper 1
The electron arrangement of ions Q2- and R3+ are as 2, 8, 8, and 2,8respectively.
(a) Write the electron arrangement of the elements Q and R (2marks)
____________________________________________________________________________________
____________________________________________________________________________________
(b) Write the formula of the compound that would be formed between Q and R (1mark)
____________________________________________________________________________________
____________________________________________________________________________________
3 marks
Explain why a high temperature is required for Nitrogen to react with oxygen
____________________________________________________________________________________
____________________________________________________________________________________
____________________________________________________________________________________
1 marks
Give one advantage and one disadvantage of using petrol containing tetraethyl lead in motor vehicles
____________________________________________________________________________________
____________________________________________________________________________________
____________________________________________________________________________________
2 marks
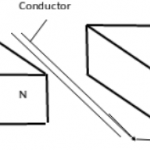
The diagram below is a cross section of a dry cell. Study it and answer the questions that follow.
(i) Write the equation for the reaction in which electrons are produced. (1mark)
____________________________________________________________________________________
____________________________________________________________________________________
(ii) The Zinc can is lined with Ammonium Chloride and Zinc Chloride paste. What would happen if the
mixture was to become dry? Give reason. (2marks)
____________________________________________________________________________________
____________________________________________________________________________________
____________________________________________________________________________________
2 marks
The graph below shows the behavior of a fixed mass of a gas at constant temperature.
(a) What is the relationship between the volume and the pressure of the gas ? (1mark)
____________________________________________________________________________________
____________________________________________________________________________________
(b) 1500cm3 of nitrogen gas at one atmosphere were compressed to two atmospheres at constant
temperature . Calculate the volume occupied by the nitrogen gas. (2marks)
____________________________________________________________________________________
____________________________________________________________________________________
3 marks
The table below gives some properties of three elements X,Yand Z.
| ELEMENT | Atomic No. | Meeting point(oC) | Boiling Point (oC) |
| X | 53 | 114 | 184 |
| Y | 35 | -7 | 58.8 |
| Z | 17 | -101 | -34.7 |
(a) Which element is in liquid form at room temperature? Give reason. (1mark)
____________________________________________________________________________________
____________________________________________________________________________________
(b) Explain why the boiling point of element X is higher than that of element Z. (2marks)
____________________________________________________________________________________
____________________________________________________________________________________
____________________________________________________________________________________
3 marks
The diagram below is a set up for the laboratory preparation of dry oxygen gas.
(a) Name:
I. Liquid Y (1 Mark)
_________________________________________________________________________________
II. Liquid X
_________________________________________________________________________________
(b) Write an equation for the reaction that took place in the flask. (1mark)
_________________________________________________________________________________
(c) Complete the diagram to show how dry oxygen can be collected. (1mark)
3 marks
Use the information below and answer the questions that follow .The letters are not the actual symbols of the elements.
(a) Calculate the E θ
value for the electrochemical cell represented below. (1mark)
(b) Arrange the elements in order of reactivity starting with the least reactive. (1mark)
____________________________________________________________________________________
____________________________________________________________________________________
(c) Explain if it would be advisable to store element G in a solution containing E2+ Ions. (1mark)
____________________________________________________________________________________
____________________________________________________________________________________
3 marks
The set up below was used to electrolyze molten lead (II) bromide.
(a) State the observation that was made at the anode during electrolysis. (1mark)
___________________________________________________________________________________
___________________________________________________________________________________
(b) A current of 2.5A was passed for 30 minutes. Calculate the mass of lead that was deposited
(2marks)
___________________________________________________________________________________
___________________________________________________________________________________
3 marks
When wood is burnt a grey powder called ash remains. The ash is stirred with water and filtered to form a colourless solution.
(a)What is the main component of the colorless solution? Give a reason. (2marks)
___________________________________________________________________________________
___________________________________________________________________________________
(b) State the observation that would be made if methyl orange indicator was passed through the solution of ash. (1mark)
___________________________________________________________________________________
___________________________________________________________________________________
3 marks
The elements A and B have the following properties
Element Mass No. Atomic No.
A 37 17
B 37 18
C
(a) When the isotope A was bombarded with a neutron, an isotope C was formed .Fill in the table to
show the properties of element C (1mark)
___________________________________________________________________________________
___________________________________________________________________________________
(b)Write an equation for the reaction between isotope B and Beta particles (1mark)
____________________________________________________________________________________
____________________________________________________________________________________
(c) State one use of radioisotopes in medicine. (1mark)
____________________________________________________________________________________
____________________________________________________________________________________
3 marks
When Carbon (IV) oxide gas was passed through aqueous calcium hydroxide a white suspension was
formed.
(a) Write an equation for the reaction that took place. (1mark)
____________________________________________________________________________________
____________________________________________________________________________________
(b) State and explain the changes that took place when excess Carbon (IV) Oxide was bubbledthrough the white suspension . (2marks)
____________________________________________________________________________________
____________________________________________________________________________________
____________________________________________________________________________________
3 marks
Excess Carbon (II) Oxide was passed over a heated sample of an oxide of iron as shown in the diagram below. Study the diagram and the data and use it to answer the questions that follow.
Mass of empty dish =6.72g
Mass of empty dish + oxide of iron =9.04g
Mass of empty dish + residue=8.40g
(a) Determine the formula of the oxide of iron given that the relative formula mass of oxide of
Iron = 232, Fe = 56.0, O=16.0 (2marks)
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
(b) Write an equation for the reaction which took place in the dish (1mark)
____________________________________________________________________________________
____________________________________________________________________________________
____________________________________________________________________________________
3 marks
The products of a burning candle were passed through a tube containing calcium oxide as shown in
the diagram below.
(a) Write two chemical equations for the reactions that took place in tube P. (2marks)
___________________________________________________________________________________
___________________________________________________________________________________
(b) Name two gases that came out through tube R. (1mark)
____________________________________________________________________________________
____________________________________________________________________________________
3 marks
Study the scheme below and answer the questions that follow.
(a)Identify substance E (1mark)
___________________________________________________________________________________
___________________________________________________________________________________
__________________________________________________________________________________
(b)Write an equation for the reaction in Step (II) that produces solid F (1mark)
_________________________________________________________________________________
_________________________________________________________________________________
2 marks
The elements nitrogen, phosphorus and potassium are essential for plant growth. Phosphorus in the
fertilizer may be in the form of ammonium phosphate. Calculate the mass of nitrogen present if a 25kg bag contained pure ammonium phosphate.
(NH4)2 HPO4 (N=14.0, H=1.0, P=31.0, O=16.0)
_________________________________________________________________________________
_________________________________________________________________________________
_________________________________________________________________________________
_________________________________________________________________________________
2 marks
The flow chart below shows the processes involved in the industrial extraction of zinc metal.
(a) Name the ore from which zinc is extracted on the above diagram. (1mark)
_________________________________________________________________________________
_________________________________________________________________________________
(b)Write the equation of the reaction taking place in Unit I (1mark)
_________________________________________________________________________________
_________________________________________________________________________________
(c) Name two uses of zinc metal. (1mark)
_________________________________________________________________________________
_________________________________________________________________________________
_________________________________________________________________________________
3 marks
A weighed sample of crystalline sodium carbonate (Na2CO3 .nH2O) was heated in a crucible until there was no further change in mass .The mass of the sample reduced by 14.5%. Calculate the number of moles (n) of the water of crystallization.
_________________________________________________________________________________
_________________________________________________________________________________
_________________________________________________________________________________
_________________________________________________________________________________
2 marks
(a) Describe how you would prepare crystals of sodium nitrate starting with 200cm3
of 2M sodium
hydroxide (2marks)
_________________________________________________________________________________
_________________________________________________________________________________
_________________________________________________________________________________
_________________________________________________________________________________
(b)Write an equation for the reaction that takes place when a solid sample of sodium nitrate is heated.
(1mark)
_________________________________________________________________________________
_________________________________________________________________________________
3 marks
The structure below represents a sweet smelling compound.
Give the names of the two organic compounds that can be used to prepare this compound in the
laboratory.
_________________________________________________________________________________
_________________________________________________________________________________
_________________________________________________________________________________
_________________________________________________________________________________
2 marks
Magnesium reacts with both concentrated and dilute acid. Write the equations for the two reactions.
_________________________________________________________________________________
_________________________________________________________________________________
_________________________________________________________________________________
_________________________________________________________________________________
2 marks
The graph below shows how the PH value of soil in a farm changed over a period of time.
(a) Describe how the PH of the soil can be determined. (2marks)
_________________________________________________________________________________
_________________________________________________________________________________
_________________________________________________________________________________
_________________________________________________________________________________
(b) State one factor that may have been responsible for the change in the soil PH in the time interval AB.
(1mark)
_________________________________________________________________________________
_________________________________________________________________________________
3 marks
A student put calcium carbonate and calcium hydrogen carbonate in separate test tubes and performed the tests as shown in the table below. Complete the table by giving the expected observations.
Salt Adding water Heating
Calcium Carbonate
Calcium hydrogen carbonate
2 marks
A mixture contains Iron (III) Chloride, calcium chloride and iron filings. Describe how one can separate and recover the substances in the mixture.
_________________________________________________________________________________
_________________________________________________________________________________
_________________________________________________________________________________
_________________________________________________________________________________
3 marks
The structure below represents two cleansing agents A and B. Which cleansing agent would be suitable for washing in water containing calcium chloride? Give a reason.
_________________________________________________________________________________
_________________________________________________________________________________
_________________________________________________________________________________
2 marks
Study the diagram below and answer the questions that follow.
(a) What do ∆H1 and ∆H2 represent. (2marks)
____________________________________________________________________________________
____________________________________________________________________________________
____________________________________________________________________________________
(b) Write an expression to show the relationship between ∆H1, ∆H2 and ∆H3. (1mark)
____________________________________________________________________________________
____________________________________________________________________________________
____________________________________________________________________________________
3 marks
Study the diagram below and use it to answer the questions that follow.
(a) Name two reagents that are reacted to produce both Carbon (IV) Oxide and Carbon (II) Oxide.
(1mark)
____________________________________________________________________________________
____________________________________________________________________________________
(b) Write the equation for the reactions that took place in the wash bottle. (1mark)
____________________________________________________________________________________
____________________________________________________________________________________
(c) Give a reason why Carbon (II) Oxide is not easily detected. (1mark)
____________________________________________________________________________________
____________________________________________________________________________________
3 marks
When a few drops of ammonia solution were added to Copper (II) Nitrate solution, a light blue
precipitate was formed. On addition of more aqueous ammonia a deep blue solution was formed.
Identify the substances responsible for the:
(a) Light blue precipitate. (1mark)
____________________________________________________________________________________
(b) Deep blue solution. (1mark)
____________________________________________________________________________________
2 marks
Study the flow chart below and answer the questions that follow.
a) Identify solution Q. (1mark)
____________________________________________________________________________________
____________________________________________________________________________________
b) Write an equation for the reaction that took place in step II. (1mark)
____________________________________________________________________________________
____________________________________________________________________________________
c) State one commercial use of gas T. (1mark)
____________________________________________________________________________________
____________________________________________________________________________________
3 marks
How does pH value of 0.1M potassium hydroxide solution compare with that of 0.1M aqueous
Ammonia? Explain
____________________________________________________________________________________
____________________________________________________________________________________
2 marks
During the manufacture of rubber raw rubber is heated with sulphur, carbon, phosphorus and manganese
i) What name is given to this process. (1mark)
____________________________________________________________________________________
____________________________________________________________________________________
ii) Explain why the process is necessary. (2marks)
____________________________________________________________________________________
____________________________________________________________________________________
3 marks